Structure ( IF 4.4 ) Pub Date : 2020-07-30 , DOI: 10.1016/j.str.2020.07.005 Alfred C Chin 1 , Remy A Yovanno 2 , Tyler J Wied 2 , Ariel Gershman 2 , Albert Y Lau 2
|
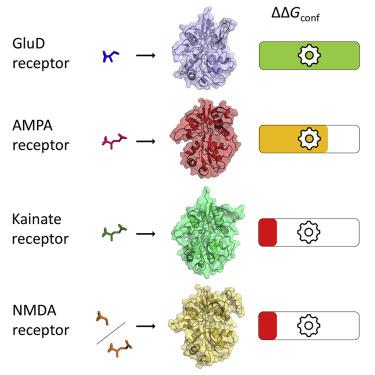
Despite their classification as ionotropic glutamate receptors, GluD receptors are not functional ligand-gated ion channels and do not bind glutamate. GluD2 receptors bind D-serine and coordinate transsynaptic complexes that regulate synaptic plasticity. Instead of opening the ion channel pore, mechanical tension produced from closure of GluD2 ligand-binding domains (LBDs) drives conformational rearrangements for non-ionotropic signaling. We report computed conformational free energy landscapes for the GluD2 LBD in apo and D-serine-bound states. Unexpectedly, the conformational free energy associated with GluD2 LBD closure upon D-serine binding is greater than that for AMPA, NMDA, and kainate receptor LBDs upon agonist binding. This excludes insufficient force generation as an explanation for lack of ion channel activity in GluD2 receptors and suggests that non-ionotropic conformational rearrangements do more work than pore opening. We also report free energy landscapes for GluD2 LBD harboring a neurodegenerative mutation and demonstrate selective stabilization of closed conformations in the apo state.
中文翻译:

D-丝氨酸有效驱动离子型谷氨酸受体 GluD2 中的配体结合域关闭。
尽管它们被归类为离子型谷氨酸受体,但 GluD 受体不是功能性配体门控离子通道,也不与谷氨酸结合。GluD2 受体结合 D-丝氨酸并协调调节突触可塑性的跨突触复合物。GluD2 配体结合域 (LBD) 的闭合产生的机械张力不是打开离子通道孔,而是驱动非离子性信号的构象重排。我们报告了载脂蛋白和 D 丝氨酸结合状态下 GluD2 LBD 的计算构象自由能景观。出乎意料的是,与 D-丝氨酸结合时与 GluD2 LBD 闭合相关的构象自由能大于激动剂结合时 AMPA、NMDA 和红藻氨酸受体 LBD 的构象自由能。这排除了作为 GluD2 受体缺乏离子通道活性的解释的力产生不足,并表明非离子型构象重排比开孔更有效。我们还报告了含有神经退行性突变的 GluD2 LBD 的自由能景观,并证明了 apo 状态下闭合构象的选择性稳定。











































 京公网安备 11010802027423号
京公网安备 11010802027423号