Molecular Cell ( IF 16.0 ) Pub Date : 2020-07-30 , DOI: 10.1016/j.molcel.2020.07.019 Dan Zhao 1 , Chen-Xi Zou 2 , Xiao-Man Liu 1 , Zhao-Di Jiang 1 , Zhong-Qiu Yu 1 , Fang Suo 1 , Tong-Yang Du 2 , Meng-Qiu Dong 3 , Wanzhong He 1 , Li-Lin Du 3
|
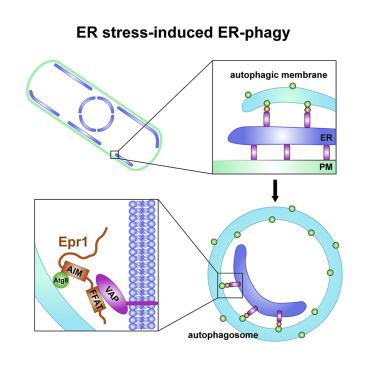
Autophagic degradation of the endoplasmic reticulum (ER-phagy) is triggered by ER stress in diverse organisms. However, molecular mechanisms governing ER stress-induced ER-phagy remain insufficiently understood. Here we report that ER stress-induced ER-phagy in the fission yeast Schizosaccharomyces pombe requires Epr1, a soluble Atg8-interacting ER-phagy receptor. Epr1 localizes to the ER through interacting with integral ER membrane proteins VAPs. Bridging an Atg8-VAP association is the main ER-phagy role of Epr1, as it can be bypassed by an artificial Atg8-VAP tether. VAPs contribute to ER-phagy not only by tethering Atg8 to the ER membrane, but also by maintaining the ER-plasma membrane contact. Epr1 is upregulated during ER stress by the unfolded protein response (UPR) regulator Ire1. Loss of Epr1 reduces survival against ER stress. Conversely, increasing Epr1 expression suppresses the ER-phagy defect and ER stress sensitivity of cells lacking Ire1. Our findings expand and deepen the molecular understanding of ER-phagy.
中文翻译:

UPR诱导的可溶性ER-Phagy受体与VAP共同作用以赋予ER压力抗性。
内质网(ER-噬菌体)的自噬降解是由多种生物体中的内质网应激引起的。然而,控制内质网应激诱导的内质网吞噬的分子机制仍知之甚少。在这里,我们报道裂变酵母粟酒裂殖酵母中的内质网应激诱导内质网吞噬需要Epr1,一种可与Atg8相互作用的可溶性ER吞噬受体。Epr1通过与完整的ER膜蛋白VAP相互作用而定位于ER。桥接Atg8-VAP关联是Epr1的主要ER吞噬作用,因为它可以被人工Atg8-VAP系链绕开。VAP不仅通过将Atg8束缚在ER膜上,而且还通过维持ER-质膜的接触来促进ER吞噬。Epr1在内质网应激期间被未折叠的蛋白应答(UPR)调节剂Ire1上调。Epr1的丢失会降低抵抗ER应激的存活率。相反,增加Epr1表达可抑制缺少Ire1的细胞的ER吞噬缺陷和ER应激敏感性。我们的发现扩展并加深了对ER吞噬的分子理解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号