Journal of Advanced Research ( IF 11.4 ) Pub Date : 2020-07-30 , DOI: 10.1016/j.jare.2020.07.016 Weijie Chen 1 , Peifen Yao 1 , Chi Teng Vong 1 , Xiuzhu Li 1 , Zhejie Chen 1 , Jianbo Xiao 1 , Shengpeng Wang 1 , Yitao Wang 1
|
Background
Ginseng has a long history of widespread use and remarkable effects as traditional medicine, adjuvant and dietary supplement. The therapeutic value, diverse functionalities and rapid development of ginseng have driven a significant increase in the number of ginseng clinical trials, ranging from its use in various ailments, formulation to safety concerns. Despite the persistent interest in ginseng clinical research, the medical effectiveness of ginseng is inconclusive and there is a lack of bibliometric analysis of the hundreds of ginseng clinical trials.
Aim of Review
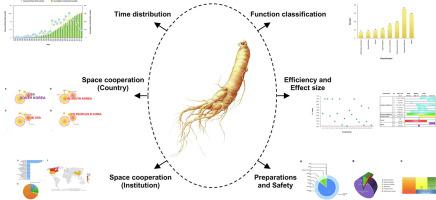
This review aims to provide an extensive overview of ginseng clinical trials over the past 40 years (1979-2018) in combination with a qualitative and quantitative analysis. The annual clinical trial analysis of time distribution, country and institution network analysis for space cooperation, statistical analysis for various functions, as well as efficiency and effect size were performed for global ginseng clinical trials. Besides, preparation categories, administration routes, and the safety of ginseng clinical trials were also investigated.
Key Scientific Concepts of Review
The 40-year journey of ginseng clinical trials has experienced emerging, boom, and stable or transitional stages. The global network of ginseng clinical trials has relevant regional distribution in Asia, North America and Europe. South Korea makes a great contribution to building up large research clusters and strong cooperation links. Universities are the key contributors to ginseng clinical trials. The development of ginseng products could be focused on the clinical trial in diseases with higher effectiveness or effect size, such as sexual function and cognitive & behavior and require rigorous investigations and evidence to evaluate safety. More attention should be paid to different effects from different preparations. We believe this review will provide new insights into the understanding of global ginseng clinical trials and identifies potential future perspectives for research and development of ginseng.
中文翻译:

人参:40年全球临床试验历程的文献计量分析
背景
人参作为传统药物、佐剂和膳食补充剂,广泛使用历史悠久,功效显着。人参的治疗价值、多样化的功能和快速发展推动了人参临床试验数量的显着增加,从其在各种疾病中的用途、配方到安全性问题。尽管人们对人参临床研究的兴趣持续存在,但人参的医疗功效尚无定论,并且缺乏对数百项人参临床试验的文献计量分析。
审查目的
本综述旨在结合定性和定量分析,对过去 40 年(1979-2018)的人参临床试验进行广泛概述。对全球人参临床试验进行年度时间分布的临床试验分析、空间合作的国家和机构网络分析、各项功能的统计分析以及效率和效应大小。此外,还对人参的制剂类别、给药途径以及人参临床试验的安全性进行了调查。
审查的关键科学概念
人参临床试验40年的历程,经历了新兴、繁荣、稳定或过渡阶段。全球人参临床试验网络在亚洲、北美和欧洲都有相关区域分布。韩国为建立大型研究集群和强有力的合作联系做出了巨大贡献。大学是人参临床试验的主要贡献者。人参产品的开发可侧重于有效性或效应量较高的疾病的临床试验,例如性功能、认知和行为,并需要严格的调查和证据来评估安全性。应更多关注不同制剂的不同效果。我们相信这篇综述将为了解全球人参临床试验提供新的见解,并确定人参研究和开发的潜在未来前景。










































 京公网安备 11010802027423号
京公网安备 11010802027423号