Chem ( IF 19.1 ) Pub Date : 2020-07-30 , DOI: 10.1016/j.chempr.2020.07.003 Chao Jiang , Lei Wang , Honggang Zhang , Pinhong Chen , Yin-Long Guo , Guosheng Liu
|
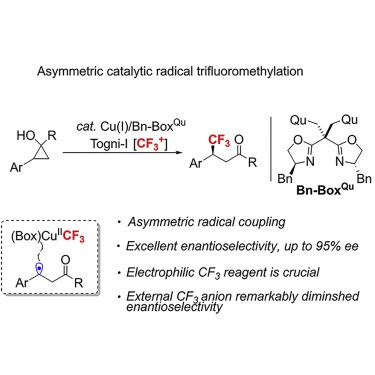
The asymmetric trifluoromethylation of aryl-substituted cyclopropanols via a radical ring-opening pathway is reported herein, which provides an easy and straightforward access to structurally diverse β-CF3 ketones in good yields and excellent enantioselectivities under very mild conditions. Critical to the success of the copper-catalyzed radical relay is that a benzylic radical intermediate can be enantioselectively trapped by reactive (L∗)CuIICF3. In addition, a novel quinolinyl-containing bisoxazoline ligand plays a significant role in the asymmetric trifluoromethylation.
中文翻译:

通过环丙醇的开环对苯氧基的对映选择性铜催化三氟甲基化
本文报道了经由自由基开环途径的芳基取代的环丙醇的不对称三氟甲基化,其提供了在非常温和的条件下容易且直接地以良好的产率和优异的对映选择性接近结构多样的β- CF 3酮。铜催化自由基继电器成功的关键在于,苄基自由基中间体可以被反应性(L ∗)Cu II CF 3对映选择性地捕获。另外,新型的含喹啉基的双恶唑啉配体在不对称三氟甲基化中起重要作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号