当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective copper-catalysed defluorosilylation of trifluoro-methylated alkenes with silylboronates
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-07-29 , DOI: 10.1039/d0qo00773k Pan Gao 1, 2, 3, 4, 5 , Liuzhou Gao 3, 4, 6, 7, 8 , Longlong Xi 1, 2, 3, 4, 5 , Zedong Zhang 1, 2, 3, 4, 5 , Shuhua Li 3, 4, 6, 7, 8 , Zhuangzhi Shi 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-07-29 , DOI: 10.1039/d0qo00773k Pan Gao 1, 2, 3, 4, 5 , Liuzhou Gao 3, 4, 6, 7, 8 , Longlong Xi 1, 2, 3, 4, 5 , Zedong Zhang 1, 2, 3, 4, 5 , Shuhua Li 3, 4, 6, 7, 8 , Zhuangzhi Shi 1, 2, 3, 4, 5
Affiliation
|
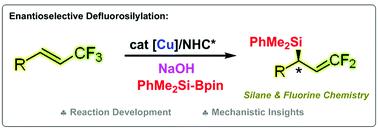
An efficient method for the construction of gem-difluoroallylsilanes with high enantiomeric excess via a copper-catalysed defluorosilylation of trifluoromethylated alkenes with silylboronates is described. The key to this high enantioselectivity is the careful selection of NaOH as the base and using a triazolium-based chiral N-heterocyclic carbene (NHC) as a ligand in the presence of a copper catalyst. The reaction conditions are mild, and excellent functional group compatibility is observed. This strategy addresses the limitations of the previously described base-mediated defluorosilylation under transition metal-free conditions, which can lead to erosion of the enantioselectivity. The synthetic utilities of the obtained gem-difluoroallylsilanes are also presented. Computational and experimental data suggest that the reaction proceeds through the enantioselective insertion of silyl-Cu/NHC species into the double bond of trifluormethyl alkenes and the Cu-mediated β-F elimination steps.
中文翻译:

三氟甲基化烯烃与甲硅烷基硼酸酯的对映选择性铜催化脱氟硅烷化
描述了通过三氟甲基化的烯烃与甲硅烷基硼酸酯的铜催化的脱氟甲硅烷基化来构建具有高对映体过量的宝石-二氟烯丙基硅烷的有效方法。这种高对映选择性的关键是在铜催化剂存在下,精心选择NaOH作为碱,并使用基于三唑鎓的手性N-杂环卡宾(NHC)作为配体。反应条件温和,观察到优异的官能团相容性。该策略解决了在无过渡金属的条件下先前描述的碱介导的脱氟甲硅烷基化的局限性,这可能导致对映选择性的侵蚀。所得宝石的合成用途还提出了-二氟烯丙基硅烷。计算和实验数据表明,该反应通过将甲硅烷基-Cu / NHC物种对映选择性插入三氟甲基烯烃的双键中以及Cu介导的β-F消除步骤来进行。
更新日期:2020-09-16
中文翻译:

三氟甲基化烯烃与甲硅烷基硼酸酯的对映选择性铜催化脱氟硅烷化
描述了通过三氟甲基化的烯烃与甲硅烷基硼酸酯的铜催化的脱氟甲硅烷基化来构建具有高对映体过量的宝石-二氟烯丙基硅烷的有效方法。这种高对映选择性的关键是在铜催化剂存在下,精心选择NaOH作为碱,并使用基于三唑鎓的手性N-杂环卡宾(NHC)作为配体。反应条件温和,观察到优异的官能团相容性。该策略解决了在无过渡金属的条件下先前描述的碱介导的脱氟甲硅烷基化的局限性,这可能导致对映选择性的侵蚀。所得宝石的合成用途还提出了-二氟烯丙基硅烷。计算和实验数据表明,该反应通过将甲硅烷基-Cu / NHC物种对映选择性插入三氟甲基烯烃的双键中以及Cu介导的β-F消除步骤来进行。











































 京公网安备 11010802027423号
京公网安备 11010802027423号