当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Usnic Acid Conjugates with Monoterpenoids as Potent Tyrosyl-DNA Phosphodiesterase 1 Inhibitors.
Journal of Natural Products ( IF 3.3 ) Pub Date : 2020-07-28 , DOI: 10.1021/acs.jnatprod.9b01089 Olga Luzina 1 , Alexander Filimonov 1, 2 , Alexandra Zakharenko 3 , Arina Chepanova 3 , Olga Zakharova 3 , Ekaterina Ilina 3 , Nadezhda Dyrkheeva 3 , Galina Likhatskaya 4 , Nariman Salakhutdinov 1, 2 , Olga Lavrik 2, 3
Journal of Natural Products ( IF 3.3 ) Pub Date : 2020-07-28 , DOI: 10.1021/acs.jnatprod.9b01089 Olga Luzina 1 , Alexander Filimonov 1, 2 , Alexandra Zakharenko 3 , Arina Chepanova 3 , Olga Zakharova 3 , Ekaterina Ilina 3 , Nadezhda Dyrkheeva 3 , Galina Likhatskaya 4 , Nariman Salakhutdinov 1, 2 , Olga Lavrik 2, 3
Affiliation
|
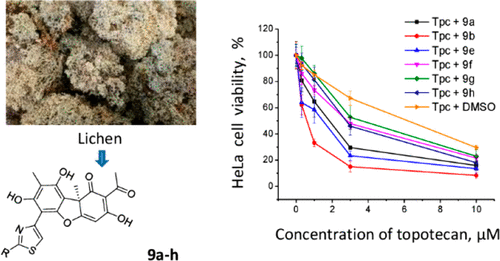
Hybrid molecules created from different pharmacophores of natural and synthetic equivalents are successfully used in pharmaceutical practice. One promising target for anticancer therapy is tyrosyl-DNA phosphodiesterase 1 (Tdp1) because it can repair DNA lesions caused by DNA-topoisomerase 1 (Top1) inhibitors, resulting in drug resistance. In this study, new hybrid compounds were synthesized by combining the pharmacophoric moiety of a set of natural compounds with inhibitory properties against Tdp1, particularly, phenolic usnic acid and a set of different monoterpenoid fragments. These fragments were connected through a hydrazinothiazole linker. The inhibitory properties of the new compounds mainly depended on the structure of the terpenoid moieties. The two most potent compounds, 9a and 9b, were synthesized from citral and citronellal, which contain acyclic fragments with IC50 values in the range of 10–16 nM. Some synthesized derivatives showed low cytotoxicity against HeLa cells and increased the effect of the Top1 inhibitor topotecan in vitro by three to seven times. These derivatives may be considered as potential agents for the development of anticancer therapies when combined with Top1 inhibitors.
中文翻译:

松萝酸与单萜类化合物作为强效酪氨酰 DNA 磷酸二酯酶 1 抑制剂。
由天然和合成等价物的不同药效团产生的混合分子已成功用于制药实践。抗癌治疗的一个有希望的靶点是酪氨酰 DNA 磷酸二酯酶 1 (Tdp1),因为它可以修复由 DNA 拓扑异构酶 1 (Top1) 抑制剂引起的 DNA 损伤,从而导致耐药性。在这项研究中,通过结合一组具有 Tdp1 抑制特性的天然化合物的药效部分,特别是酚松香酸和一组不同的单萜类片段,合成了新的杂化化合物。这些片段通过肼基噻唑接头连接。新化合物的抑制特性主要取决于萜类化合物的结构。两种最有效的化合物,9a和9b, 由柠檬醛和香茅醛合成,其中包含 IC 50值在 10-16 nM 范围内的无环片段。一些合成的衍生物对 HeLa 细胞显示出低细胞毒性,并将 Top1 抑制剂拓扑替康的体外作用提高了三到七倍。当与 Top1 抑制剂联合使用时,这些衍生物可能被认为是开发抗癌疗法的潜在药物。
更新日期:2020-08-28
中文翻译:

松萝酸与单萜类化合物作为强效酪氨酰 DNA 磷酸二酯酶 1 抑制剂。
由天然和合成等价物的不同药效团产生的混合分子已成功用于制药实践。抗癌治疗的一个有希望的靶点是酪氨酰 DNA 磷酸二酯酶 1 (Tdp1),因为它可以修复由 DNA 拓扑异构酶 1 (Top1) 抑制剂引起的 DNA 损伤,从而导致耐药性。在这项研究中,通过结合一组具有 Tdp1 抑制特性的天然化合物的药效部分,特别是酚松香酸和一组不同的单萜类片段,合成了新的杂化化合物。这些片段通过肼基噻唑接头连接。新化合物的抑制特性主要取决于萜类化合物的结构。两种最有效的化合物,9a和9b, 由柠檬醛和香茅醛合成,其中包含 IC 50值在 10-16 nM 范围内的无环片段。一些合成的衍生物对 HeLa 细胞显示出低细胞毒性,并将 Top1 抑制剂拓扑替康的体外作用提高了三到七倍。当与 Top1 抑制剂联合使用时,这些衍生物可能被认为是开发抗癌疗法的潜在药物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号