当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis, and biological evaluation of piperidinyl-substituted [1,2,4]triazolo[1,5-a]pyrimidine derivatives as potential anti-HIV-1 agents with reduced cytotoxicity.
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2020-07-28 , DOI: 10.1111/cbdd.13760 Boshi Huang 1 , Dongwei Kang 1 , Ye Tian 1 , Dirk Daelemans 2 , Erik De Clercq 2 , Christophe Pannecouque 2 , Peng Zhan 1 , Xinyong Liu 1
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2020-07-28 , DOI: 10.1111/cbdd.13760 Boshi Huang 1 , Dongwei Kang 1 , Ye Tian 1 , Dirk Daelemans 2 , Erik De Clercq 2 , Christophe Pannecouque 2 , Peng Zhan 1 , Xinyong Liu 1
Affiliation
|
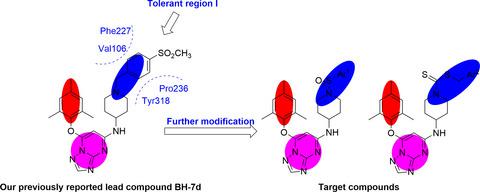
Taking the previously reported compound BH‐7d as the lead, we designed and synthesized a series of piperidinyl‐substituted [1,2,4]triazolo[1,5‐a]pyrimidines, and their anti‐HIV activities as well as cytotoxicities were evaluated. Several compounds exhibited moderate anti‐HIV (IIIB) potency, among which 2b was the most active one (EC50 = 4.29 μM). Structure–activity relationships derived from the antiretroviral results were analyzed. Additionally, most compounds demonstrated reduced cytotoxicity (CC50 > 200 μM) compared with those of BH‐7d and etravirine. Molecular docking study further revealed the binding conformation of 2b in the binding pocket of HIV‐1 reverse transcriptase.
中文翻译:

哌啶基取代的 [1,2,4] 三唑并 [1,5-a] 嘧啶衍生物作为具有降低细胞毒性的潜在抗 HIV-1 药物的设计、合成和生物学评价。
以先前报道的化合物BH-7d为先导,我们设计合成了一系列哌啶基取代的[1,2,4]三唑并[1,5-a]嘧啶类化合物,其抗HIV活性和细胞毒性均在评估。几种化合物表现出中等的抗 HIV (IIIB) 效力,其中2b是最活跃的一种 (EC 50 = 4.29 μM)。分析了源自抗逆转录病毒结果的构效关系。此外, 与BH-7d和依曲韦林相比,大多数化合物表现出降低的细胞毒性(CC 50 > 200 μM)。分子对接研究进一步揭示了HIV-1 逆转录酶结合口袋中2b的结合构象。
更新日期:2020-07-28
中文翻译:

哌啶基取代的 [1,2,4] 三唑并 [1,5-a] 嘧啶衍生物作为具有降低细胞毒性的潜在抗 HIV-1 药物的设计、合成和生物学评价。
以先前报道的化合物BH-7d为先导,我们设计合成了一系列哌啶基取代的[1,2,4]三唑并[1,5-a]嘧啶类化合物,其抗HIV活性和细胞毒性均在评估。几种化合物表现出中等的抗 HIV (IIIB) 效力,其中2b是最活跃的一种 (EC 50 = 4.29 μM)。分析了源自抗逆转录病毒结果的构效关系。此外, 与BH-7d和依曲韦林相比,大多数化合物表现出降低的细胞毒性(CC 50 > 200 μM)。分子对接研究进一步揭示了HIV-1 逆转录酶结合口袋中2b的结合构象。











































 京公网安备 11010802027423号
京公网安备 11010802027423号