当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Light-Controlled Regioselective Synthesis of Fullerene Bis-Adducts.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-07-28 , DOI: 10.1002/anie.202009235 Luka Ðorđević 1, 2, 3 , Lorenzo Casimiro 4, 5, 6 , Nicola Demitri 7 , Massimo Baroncini 4, 8 , Serena Silvi 4, 5 , Francesca Arcudi 1, 2 , Alberto Credi 4, 9 , Maurizio Prato 1, 10, 11
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-07-28 , DOI: 10.1002/anie.202009235 Luka Ðorđević 1, 2, 3 , Lorenzo Casimiro 4, 5, 6 , Nicola Demitri 7 , Massimo Baroncini 4, 8 , Serena Silvi 4, 5 , Francesca Arcudi 1, 2 , Alberto Credi 4, 9 , Maurizio Prato 1, 10, 11
Affiliation
|
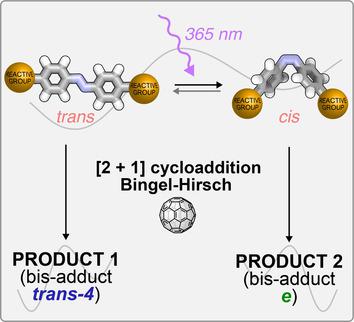
Multi‐functionalization and isomer‐purity of fullerenes are crucial tasks for the development of their chemistry in various fields. In both current main approaches—tether‐directed covalent functionalization and supramolecular masks—the control of regioselectivity requires multi‐step synthetic procedures to prepare the desired tether or mask. Herein, we describe light‐responsive tethers, containing an azobenzene photoswitch and two malonate groups, in the double cyclopropanation of [60]fullerene. The formation of the bis‐adducts and their spectroscopic and photochemical properties, as well as the effect of azobenzene photoswitching on the regiochemistry of the bis‐addition, have been studied. The behavior of the tethers depends on the geometry of the connection between the photoactive core and the malonate moieties. One tether lead to a strikingly different adduct distribution for the E and Z isomers, indicating that the covalent bis‐functionalization of C60 can be controlled by light.
中文翻译:

富勒烯双加合物的光控区域选择性合成。
富勒烯的多功能化和异构体纯度是其在各个领域化学发展的关键任务。在当前的两种主要方法(系链导向的共价官能化和超分子掩膜)中,区域选择性的控制都需要多步合成程序来制备所需的系链或掩膜。在本文中,我们描述了在[60]富勒烯的双环丙烷化反应中包含偶氮苯光电开关和两个丙二酸酯基团的光响应性链。研究了双加合物的形成及其光谱和光化学性质,以及偶氮苯光开关对双加合物的区域化学的影响。系链的行为取决于光敏核与丙二酸部分之间连接的几何形状。E和Z的异构体,表明C 60的共价双官能化可以通过光控制。
更新日期:2020-07-28
中文翻译:

富勒烯双加合物的光控区域选择性合成。
富勒烯的多功能化和异构体纯度是其在各个领域化学发展的关键任务。在当前的两种主要方法(系链导向的共价官能化和超分子掩膜)中,区域选择性的控制都需要多步合成程序来制备所需的系链或掩膜。在本文中,我们描述了在[60]富勒烯的双环丙烷化反应中包含偶氮苯光电开关和两个丙二酸酯基团的光响应性链。研究了双加合物的形成及其光谱和光化学性质,以及偶氮苯光开关对双加合物的区域化学的影响。系链的行为取决于光敏核与丙二酸部分之间连接的几何形状。E和Z的异构体,表明C 60的共价双官能化可以通过光控制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号