Journal of Proteomics ( IF 2.8 ) Pub Date : 2020-07-29 , DOI: 10.1016/j.jprot.2020.103926 Progga Sen 1 , Chin Fung Kelvin Kan 1 , Amar B Singh 1 , Monica Rius 1 , Fredric B Kraemer 2 , Elizabeth Sztul 3 , Jingwen Liu 1
|
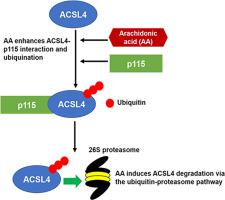
Long-chain acyl-CoA synthetase 4 (ACSL4) is an ACSL family member that exhibits unique substrate preference for arachidonic acid. ACSL4 has a functional role in hepatic lipid metabolism, and is dysregulated in non-alcoholic fatty liver disease. Our previous studies demonstrated AA-induced ACSL4 degradation via the ubiquitin-proteasomal pathway (UPP). To characterize this unique mechanism, we applied proteomic approaches coupled with LC-MS/MS and identified the intracellular general vesicular trafficking protein p115 as the prominent ACSL4 interacting protein in HepG2 cells. Importantly, we found that AA greatly enhanced p115-ACSL4 association. Combined AA treatment with p115 knockdown suggested an additive role for p115 in AA-driven ACSL4 degradation. Furthermore, in vivo studies revealed a significant upregulation of p115 protein in the liver of mice fed a high fat diet that has been previously reported to induce downregulation of ACSL4 protein expression. This new finding has revealed a novel inverse correlation between ACSL4 and p115 proteins in the liver. p115 is crucial for ER-Golgi trafficking and Golgi biogenesis. Thus far, p115 has not been reported to interact with UPP proteins nor with FA metabolism enzymes. Overall, our current study provides a novel insight into the connection between ER-Golgi trafficking and UPP machinery with p115 as a critical mediator.
Significance
ACSL4 is uniquely regulated by its own substrate AA, and in this study, we have found that AA leads to an enhanced interaction of ACSL4 with a novel interacting partner, the intracellular vesicle trafficking protein p115. The latter is crucial for Golgi biogenesis and ER-Golgi transport and is not known to be associated with the ubiquitin-proteasome machinery or protein stability regulation until now. This study is the first report of a possible coordination of the protein secretion pathway and the UPP in regulating a key metabolic enzyme. Our study lays the foundation to this unique crosstalk between the two major cellular pathways- secretion and protein degradation and opens up a new avenue to explore this partnership in controlling hepatic lipid metabolism. Overall, the complete elucidation of the AA-mediated ACSL4 regulation will help identify key targets in participating pathways that can be further studied for the development of therapeutics against diseases such as NAFLD, NASH and hepatocarcinoma, which are associated with dysregulated ACSL4 function.
中文翻译:

p115 作为一种新型 ACSL4 相互作用蛋白的鉴定及其在调节 ACSL4 降解中的作用。
长链酰基辅酶A 合成酶 4 (ACSL4) 是 ACSL 家族成员,对花生四烯酸表现出独特的底物偏好。ACSL4 在肝脏脂质代谢中具有功能性作用,并且在非酒精性脂肪肝疾病中失调。我们之前的研究表明,AA 可通过泛素-蛋白酶体途径 (UPP) 诱导 ACSL4 降解。为了表征这种独特的机制,我们应用蛋白质组学方法与 LC-MS/MS 相结合,并将细胞内一般囊泡运输蛋白 p115 鉴定为 HepG2 细胞中主要的 ACSL4 相互作用蛋白。重要的是,我们发现 AA 大大增强了 p115-ACSL4 关联。AA 处理与 p115 敲低相结合表明 p115 在 AA 驱动的 ACSL4 降解中具有附加作用。此外,体内研究表明,喂食高脂肪饮食的小鼠肝脏中 p115 蛋白显着上调,此前有报道称这种饮食会诱导 ACSL4 蛋白表达的下调。这一新发现揭示了肝脏中 ACSL4 和 p115 蛋白之间的一种新的负相关。p115 对 ER-高尔基体贩运和高尔基体生物发生至关重要。到目前为止,尚未报道 p115 与 UPP 蛋白或 FA 代谢酶相互作用。总体而言,我们目前的研究为 ER-Golgi 贩运和 UPP 机制之间的联系提供了新的见解,其中 p115 作为关键介质。p115 对 ER-高尔基体贩运和高尔基体生物发生至关重要。到目前为止,尚未报道 p115 与 UPP 蛋白或 FA 代谢酶相互作用。总体而言,我们目前的研究为 ER-Golgi 贩运和 UPP 机制之间的联系提供了新的见解,其中 p115 作为关键介质。p115 对 ER-高尔基体贩运和高尔基体生物发生至关重要。到目前为止,尚未报道 p115 与 UPP 蛋白或 FA 代谢酶相互作用。总体而言,我们目前的研究为 ER-Golgi 贩运和 UPP 机制之间的联系提供了新的见解,其中 p115 作为关键介质。
意义
ACSL4 受到其自身底物 AA 的独特调节,在本研究中,我们发现 AA 导致 ACSL4 与新的相互作用伙伴细胞内囊泡运输蛋白 p115 的相互作用增强。后者对于高尔基体生物发生和 ER-高尔基体转运至关重要,直到现在还不知道与泛素-蛋白酶体机制或蛋白质稳定性调节有关。这项研究是蛋白质分泌途径和UPP在调节关键代谢酶中可能协调的第一份报告。我们的研究为两种主要细胞途径——分泌和蛋白质降解之间的这种独特的串扰奠定了基础,并为探索这种控制肝脏脂质代谢的伙伴关系开辟了一条新途径。全面的,











































 京公网安备 11010802027423号
京公网安备 11010802027423号