当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Stabilizing Mechanism of Immobilized Metagenomic Xylanases on Bio-Based Hydrogels to Improve Utilization Performance: Computational and Functional Perspectives.
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2020-07-28 , DOI: 10.1021/acs.bioconjchem.0c00361 Shohreh Ariaeenejad 1 , Hossein Lanjanian 2 , Elaheh Motamedi 3 , Kaveh Kavousi 2 , Ali A Moosavi-Movahedi 2 , Ghasem Hosseini Salekdeh 1, 4
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2020-07-28 , DOI: 10.1021/acs.bioconjchem.0c00361 Shohreh Ariaeenejad 1 , Hossein Lanjanian 2 , Elaheh Motamedi 3 , Kaveh Kavousi 2 , Ali A Moosavi-Movahedi 2 , Ghasem Hosseini Salekdeh 1, 4
Affiliation
|
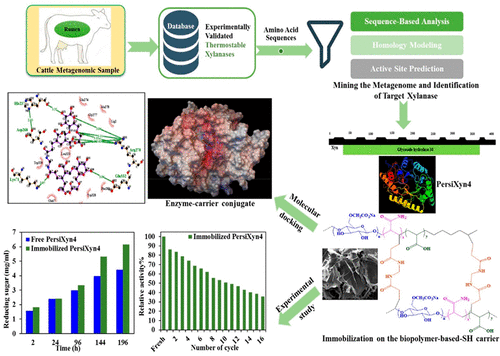
While polysaccharide-based superabsorbent hydrogels (SHs) have attracted increasing interest as proficient carriers in the enzyme immobilization, the nature of the favored interactions between the SHs and enzymes is still unclear. Herein, a combined experimental and computational study was employed to investigate the dominant parameters affecting on the stabilization of two metagenomic xylanases on the SHs. The thermostable enzymes (PersiXyn3 and PersiXyn4) with similar domains were screened, cloned, expressed, and purified from cattle rumen metagenome. Then, the enzymes were immobilized on the carboxymethyl cellulose-g-poly(acrylic acid-co-acrylamide) hydrogel which resulted in increasing their activity and stability. The carboxymethyl cellulose (CMC)-based characteristic of the hydrogel provided high numbers of H-bondings/ionic bridges, causing an improvement in the stability, hydrolysis performance, and reusability of the immobilized enzymes. More specifically, enzyme immobilization resulted in ∼40% increase in the content of the reducing sugars released after treatment of paper pulp. After 16 reuse cycles, the immobilized PersiXyn4 displayed 35.9% activity, but the immobilized PersiXyn3 retained just 8.2% of its initial activity. The comparative investigations illustrated that a higher number of positively charged amino acids in the binding site of the enzyme provided stronger electrostatic attractions between it and negative functionalities of the hydrogel. This was suggested as the main reason for the higher affinity of PersiXyn4 toward hydrogel and explained the better hydrolysis performance and reusability of the immobilized PersiXyn4 on the SH. These findings are essential for designing novel innovative SH carriers and the successful engineering of optimal enzyme assemblies through the prediction of the immobilized enzyme’s stabilities.
中文翻译:

生物基水凝胶上固定的基因组木聚糖酶稳定化机制,以提高利用性能:计算和功能的观点。
虽然基于多糖的超吸收性水凝胶(SHs)作为高效的载体固定在酶上已引起了越来越多的兴趣,但尚不清楚SHs和酶之间相互作用的性质。在本文中,结合实验和计算研究来研究影响SHs上两个宏基因组木聚糖酶稳定性的主要参数。从牛瘤胃基因组中筛选,克隆,表达和纯化具有相似结构域的热稳定酶(PersiXyn3和PersiXyn4)。然后,将酶固定在羧甲基纤维素的克-聚(丙烯酸-共-丙烯酰胺)水凝胶,从而提高了它们的活性和稳定性。水凝胶的基于羧甲基纤维素(CMC)的特性可提供大量的H键/离子桥,从而提高了固定化酶的稳定性,水解性能和可重复使用性。更具体地说,酶固定化处理纸浆后释放的还原糖含量增加了约40%。在16个重复使用周期后,固定化的PersiXyn4表现出35.9%的活性,但是固定化的PersiXyn3仅保留了其初始活性的8.2%。对比研究表明,酶结合位点中带更多正电荷的氨基酸在酶与水凝胶的负功能之间提供了更强的静电吸引力。这被认为是PersiXyn4对水凝胶具有更高亲和力的主要原因,并解释了固定化的PersiXyn4在SH上具有更好的水解性能和可重复使用性。这些发现对于设计新颖的创新SH载体以及通过预测固定化酶的稳定性成功设计最佳酶组装体至关重要。
更新日期:2020-09-16
中文翻译:

生物基水凝胶上固定的基因组木聚糖酶稳定化机制,以提高利用性能:计算和功能的观点。
虽然基于多糖的超吸收性水凝胶(SHs)作为高效的载体固定在酶上已引起了越来越多的兴趣,但尚不清楚SHs和酶之间相互作用的性质。在本文中,结合实验和计算研究来研究影响SHs上两个宏基因组木聚糖酶稳定性的主要参数。从牛瘤胃基因组中筛选,克隆,表达和纯化具有相似结构域的热稳定酶(PersiXyn3和PersiXyn4)。然后,将酶固定在羧甲基纤维素的克-聚(丙烯酸-共-丙烯酰胺)水凝胶,从而提高了它们的活性和稳定性。水凝胶的基于羧甲基纤维素(CMC)的特性可提供大量的H键/离子桥,从而提高了固定化酶的稳定性,水解性能和可重复使用性。更具体地说,酶固定化处理纸浆后释放的还原糖含量增加了约40%。在16个重复使用周期后,固定化的PersiXyn4表现出35.9%的活性,但是固定化的PersiXyn3仅保留了其初始活性的8.2%。对比研究表明,酶结合位点中带更多正电荷的氨基酸在酶与水凝胶的负功能之间提供了更强的静电吸引力。这被认为是PersiXyn4对水凝胶具有更高亲和力的主要原因,并解释了固定化的PersiXyn4在SH上具有更好的水解性能和可重复使用性。这些发现对于设计新颖的创新SH载体以及通过预测固定化酶的稳定性成功设计最佳酶组装体至关重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号