当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Potent Inhibition of Mandelate Racemase by Boronic Acids: Boron as a Mimic of a Carbon Acid Center.
Biochemistry ( IF 2.9 ) Pub Date : 2020-07-28 , DOI: 10.1021/acs.biochem.0c00478 Amar Nath Sharma 1 , Lia Grandinetti 2 , Erin R Johnson 3 , Martin St Maurice 2 , Stephen L Bearne 1, 3
Biochemistry ( IF 2.9 ) Pub Date : 2020-07-28 , DOI: 10.1021/acs.biochem.0c00478 Amar Nath Sharma 1 , Lia Grandinetti 2 , Erin R Johnson 3 , Martin St Maurice 2 , Stephen L Bearne 1, 3
Affiliation
|
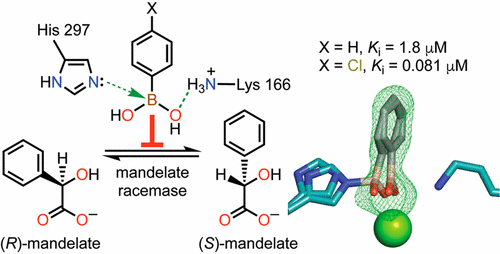
Boronic acids have been successfully employed as inhibitors of hydrolytic enzymes. Typically, an enzymatic nucleophile catalyzing hydrolysis adds to the electrophilic boron atom forming a tetrahedral species that mimics the intermediate(s)/transition state(s) for the hydrolysis reaction. We show that para-substituted phenylboronic acids (PBAs) are potent competitive inhibitors of mandelate racemase (MR), an enzyme that catalyzes a 1,1-proton transfer rather than a hydrolysis reaction. The Ki value for PBA was 1.8 ± 0.1 μM, and p-Cl-PBA exhibited the most potent inhibition (Ki = 81 ± 4 nM), exceeding the binding affinity of the substrate by ∼4 orders of magnitude. Isothermal titration calorimetric studies with the wild-type, K166M, and H297N MR variants indicated that, of the two Brønsted acid–base catalysts Lys 166 and His 297, the former made the greater contribution to inhibitor binding. The X-ray crystal structure of the MR·PBA complex revealed the presence of multiple H-bonds between the boronic acid hydroxyl groups and the side chains of active site residues, as well as formation of a His 297 Nε2–B dative bond. The dramatic upfield change in chemical shift of 27.2 ppm in the solution-phase 11B nuclear magnetic resonance spectrum accompanying binding of PBA by MR was consistent with an sp3-hybridized boron, which was also supported by density-functional theory calculations. These unprecedented findings suggest that, beyond substituting boron at carbon centers participating in hydrolysis reactions, substitution of boron at the acidic carbon center of a substrate furnishes a new approach for generating inhibitors of enzymes catalyzing the deprotonation of carbon acid substrates.
中文翻译:

硼酸对扁桃酸酯消旋酶的有效抑制作用:硼作为碳酸中心的模拟物。
硼酸已成功地用作水解酶的抑制剂。通常,酶促亲核试剂催化水解添加到亲电硼原子上,形成四面体物种,该四面体物种模仿用于水解反应的中间体/过渡态。我们表明对位取代的苯基硼酸(PBA)是扁桃酸酯消旋酶(MR)的有效竞争性抑制剂,扁桃酸酯消旋酶(MR)催化1,1-质子转移而不是水解反应。PBA的K i值为1.8±0.1μM,对-Cl-PBA表现出最强的抑制作用(K i= 81±4nM),超过底物的结合亲和力约4个数量级。用野生型,K166M和H297N MR变体进行等温滴定热研究表明,在两种布朗斯台德酸碱催化剂Lys 166和His 297中,前者对抑制剂结合的贡献更大。MR·PBA配合物的X射线晶体结构揭示了硼酸羟基与活性位点残基侧链之间存在多个H键,并形成了His 297 Nε2– B亲和键。伴随着MR与PBA结合的溶液相11 B核磁共振波谱中化学位移27.2 ppm的剧烈上场变化与sp 3一致-杂交硼,也得到密度泛函理论计算的支持。这些空前的发现表明,除了在参与水解反应的碳中心处取代硼之外,在底物的酸性碳中心处取代硼还提供了一种新的方法来产生催化碳酸底物去质子化的酶抑制剂。
更新日期:2020-08-25
中文翻译:

硼酸对扁桃酸酯消旋酶的有效抑制作用:硼作为碳酸中心的模拟物。
硼酸已成功地用作水解酶的抑制剂。通常,酶促亲核试剂催化水解添加到亲电硼原子上,形成四面体物种,该四面体物种模仿用于水解反应的中间体/过渡态。我们表明对位取代的苯基硼酸(PBA)是扁桃酸酯消旋酶(MR)的有效竞争性抑制剂,扁桃酸酯消旋酶(MR)催化1,1-质子转移而不是水解反应。PBA的K i值为1.8±0.1μM,对-Cl-PBA表现出最强的抑制作用(K i= 81±4nM),超过底物的结合亲和力约4个数量级。用野生型,K166M和H297N MR变体进行等温滴定热研究表明,在两种布朗斯台德酸碱催化剂Lys 166和His 297中,前者对抑制剂结合的贡献更大。MR·PBA配合物的X射线晶体结构揭示了硼酸羟基与活性位点残基侧链之间存在多个H键,并形成了His 297 Nε2– B亲和键。伴随着MR与PBA结合的溶液相11 B核磁共振波谱中化学位移27.2 ppm的剧烈上场变化与sp 3一致-杂交硼,也得到密度泛函理论计算的支持。这些空前的发现表明,除了在参与水解反应的碳中心处取代硼之外,在底物的酸性碳中心处取代硼还提供了一种新的方法来产生催化碳酸底物去质子化的酶抑制剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号