Journal of Structural Biology ( IF 3.0 ) Pub Date : 2020-07-28 , DOI: 10.1016/j.jsb.2020.107593 Junchao Wang 1 , Rui Xiang 1 , Rongjuan Wang 2 , Buchang Zhang 3 , Weimin Gong 4 , Jinchao Zhang 2 , Min Zhang 5 , Mingzhu Wang 1
|
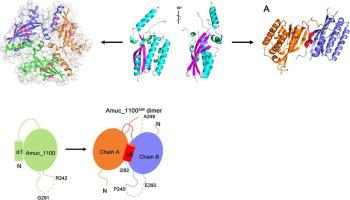
Akkermansia muciniphila is a beneficial microorganism colonized in the human gut that can reverse many intestinal metabolic-related diseases. Amuc_1100 is an outer-membrane protein of A. muciniphila. Oral administration of Amuc_1100 can reduce fat mass development, insulin resistance, and dyslipidemia in mice and activated the toll-like receptor 2 (TLR2) to regulate the immune response of the host, but the molecular mechanism remains unclear. Here we report the crystal structure of the extramembranous domain of Amuc_1100, which consists of a four-stranded antiparallel β-sheet and four α-helices. Two C-terminal helices and the four-stranded antiparallel β-sheet formed two “αββ” motifs and constituted the core domain, which shared a similar fold with type IV pili and type II Secretion system protein. Although the full-length of the extramembranous domain of Amuc_1100 existed as a monomer in solution, they formed trimer in the crystal. Elimination of the N-terminal coiled-coil helix α1 led to dimerization of Amuc_1100 both in solution and in crystal, indicating that the oligomeric state of Amuc_1100 was variable and could be influenced by α1. In addition, we identified that Amuc_1100 could directly bind human TLR2 (hTRL2) in vitro, suggesting that Amuc_1100 may serve as a new ligand for hTLR2. Dimerization of Amuc_1100 improved its hTLR2-binding affinity, suggesting that the α1-truncated Amuc_1100 could be a beneficial candidate for the development of A. muciniphila related drugs.
中文翻译:

来自 Akkermansia muciniphila 的 Amuc_1100 的可变寡聚状态。
Akkermansia muciniphila是一种寄居在人类肠道中的有益微生物,可以逆转许多肠道代谢相关疾病。Amuc_1100 是A. muciniphila的外膜蛋白. 口服Amuc_1100可以减少小鼠脂肪量的形成、胰岛素抵抗和血脂异常,并激活toll样受体2(TLR2)来调节宿主的免疫反应,但分子机制尚不清楚。在这里,我们报告了 Amuc_1100 膜外结构域的晶体结构,它由一个四链反平行 β-折叠和四个 α-螺旋组成。两个C-末端螺旋和四链反平行β-折叠形成两个“αββ”基序并构成核心域,其与IV型菌毛和II型分泌系统蛋白共享相似的折叠。虽然Amuc_1100的全长膜外结构域以单体形式存在于溶液中,但它们在晶体中形成了三聚体。消除 N 端卷曲螺旋 α1 导致 Amuc_1100 在溶液和晶体中的二聚化,表明 Amuc_1100 的寡聚状态是可变的,可能受 α1 的影响。此外,我们发现 Amuc_1100 可以直接结合人类 TLR2 (hTRL2)在体外,表明 Amuc_1100 可以作为 hTLR2 的新配体。Amuc_1100 的二聚化提高了其 hTLR2 结合亲和力,表明 α1 截短的 Amuc_1100 可能是开发A. muciniphila相关药物的有益候选物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号