Insect Biochemistry and Molecular Biology ( IF 3.2 ) Pub Date : 2020-07-28 , DOI: 10.1016/j.ibmb.2020.103362 Ibrahim A Abdulganiyyu 1 , Krzysztof Kaczmarek 2 , Janusz Zabrocki 2 , Ronald J Nachman 3 , Elisabeth Marchal 4 , Sam Schellens 4 , Heleen Verlinden 4 , Jozef Vanden Broeck 4 , Heather Marco 5 , Graham E Jackson 1
|
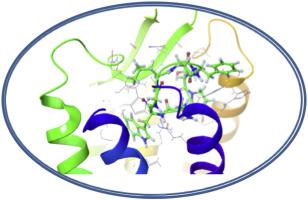
Neuropeptides belonging to the adipokinetic hormone (AKH) family elicit metabolic effects as their main function in insects, by mobilizing trehalose, diacylgycerol, or proline, which are released from the fat body into the hemolymph as energy sources for muscle contraction required for energy-intensive processes, such as locomotion. One of the AKHs produced in locusts is a decapeptide, Locmi-AKH-I (pELNFTPNWGT-NH2). A head-to-tail cyclic, octapeptide analog of Locmi-AKH-I, cycloAKH (cyclo[LNFTPNWG]) was synthesized to severely restrict the conformational freedom of the AKH structure. In vitro, cycloAKH selectively retains full efficacy on a pest insect (desert locust) AKH receptor, while showing little or no activation of the AKH receptor of a beneficial insect (honeybee). Molecular dynamic analysis incorporating NMR data indicate that cycloAKH preferentially adopts a type II β-turn under micelle conditions, whereas its linear counterpart and natural AKH adopts a type VI β-turn under similar conditions. CycloAKH, linear LNFTPNWG-NH2, and Locmi-AKH-I feature the same binding site during docking simulations with the desert locust AKH receptor (Schgr-AKHR), but differ in the details of the ligand/receptor interactions. However, cycloAKH failed to enter the binding pocket of the honeybee receptor 3D model during docking simulations. Since the locust AKH receptor has a greater tolerance than the honeybee receptor for the cyclic conformational constraint in vitro receptor assays, it could suggest a greater tolerance for a shift in the direction of the type II β turn exhibited by cycloAKH from the type VI β turn of the linear octapeptide and the native locust decapeptide AKH. Selectivity in biostable mimetic analogs could potentially be enhanced by incorporating conformational constraints that emphasize this shift. Biostable mimetic analogs of AKH offer the potential of selectively disrupting AKH-regulated processes, leading to novel, environmentally benign control strategies for pest insect populations.
中文翻译:

构象分析的环状AKH神经肽类似物,对蝗虫和蜜蜂受体具有选择性活性。
属于脂肪代谢激素(AKH)家族的神经肽通过动员海藻糖,二酰基甘油或脯氨酸来激发代谢作用,从而在昆虫中发挥主要作用,而海藻糖,二酰基甘油或脯氨酸则从脂肪体内释放到血淋巴中,作为能量密集型肌肉收缩所需的能量来源过程,例如运动。在蝗虫中产生的AKH之一是十肽Locmi-AKH-1(pELNFTPNWGT-NH 2)。合成了Locmi-AKH-1的头尾环状八肽类似物环AKH(环[LNFTPNWG]),以严格限制AKH结构的构象自由度。在体外,环AKH对害虫(沙漠蝗虫)AKH受体有选择性地保持全部功效,而对有益昆虫(蜜蜂)的AKH受体几乎没有激活。结合NMR数据的分子动力学分析表明,环AKH在胶束条件下优先采用II型β-转角,而其线性对应物和天然AKH在相似条件下采用VI型β-转角。Cyclo AKH,线性LNFTPNWG-NH 2和Locmi-AKH-1在与沙漠蝗AKH受体(Schgr-AKHR)的对接模拟过程中具有相同的结合位点,但配体/受体相互作用的细节不同。但是,环在对接模拟过程中,AKH无法进入蜜蜂受体3D模型的绑定袋。由于刺槐AKH受体在体外受体测定中对环状构象约束的耐受性高于蜜蜂受体,因此可能暗示对环AKH从VIβ型向IIβ型转弯方向变化的耐受性更高线性八肽和天然蝗虫十肽AKH的转数。通过加入强调这种变化的构象约束,可以潜在地提高生物稳定模拟物类似物的选择性。AKH的生物稳定模拟物提供了选择性破坏AKH调节过程的潜力,从而为有害生物昆虫种群带来了新颖的,环境友好的控制策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号