Cell Systems ( IF 9.0 ) Pub Date : 2020-07-28 , DOI: 10.1016/j.cels.2020.07.004 Alexander E Davies 1 , Michael Pargett 2 , Stefan Siebert 2 , Taryn E Gillies 2 , Yongin Choi 2 , Savannah J Tobin 3 , Abhineet R Ram 2 , Vaibhav Murthy 4 , Celina Juliano 2 , Gerald Quon 2 , Mina J Bissell 5 , John G Albeck 2
|
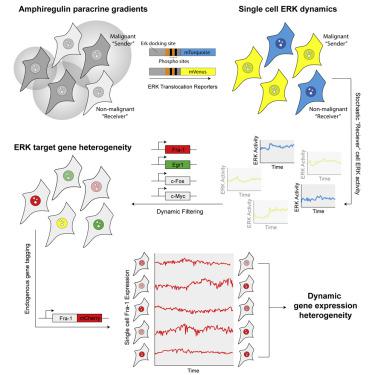
Intratumoral heterogeneity is associated with aggressive tumor behavior, therapy resistance, and poor patient outcomes. Such heterogeneity is thought to be dynamic, shifting over periods of minutes to hours in response to signaling inputs from the tumor microenvironment. However, models of this process have been inferred from indirect or post-hoc measurements of cell state, leaving the temporal details of signaling-driven heterogeneity undefined. Here, we developed a live-cell model system in which microenvironment-driven signaling dynamics can be directly observed and linked to variation in gene expression. Our analysis reveals that paracrine signaling between two cell types is sufficient to drive continual diversification of gene expression programs. This diversification emerges from systems-level properties of the EGFR-RAS-ERK signaling cascade, including intracellular amplification of amphiregulin-mediated paracrine signals and differential kinetic filtering by target genes including Fra-1, c-Myc, and Egr1. Our data enable more precise modeling of paracrine-driven transcriptional variation as a generator of gene expression heterogeneity. A record of this paper’s transparent peer review process is included in the Supplemental Information.
中文翻译:

EGFR-RAS-ERK 信号传导的系统级特性可放大局部信号以产生动态基因表达异质性。
瘤内异质性与侵袭性肿瘤行为、治疗抵抗和不良患者预后相关。这种异质性被认为是动态的,会根据肿瘤微环境的信号输入在几分钟到几小时内发生变化。然而,这一过程的模型是从细胞状态的间接或事后测量中推断出来的,导致信号驱动异质性的时间细节未定义。在这里,我们开发了一个活细胞模型系统,在该系统中可以直接观察微环境驱动的信号动力学并将其与基因表达的变化联系起来。我们的分析表明,两种细胞类型之间的旁分泌信号传导足以驱动基因表达程序的持续多样化。这种多样化源于 EGFR-RAS-ERK 信号级联的系统级特性,包括双调蛋白介导的旁分泌信号的细胞内放大以及 Fra-1、c-Myc 和 Egr1 等靶基因的差异动力学过滤。我们的数据能够对旁分泌驱动的转录变异进行更精确的建模,作为基因表达异质性的生成器。本文透明同行评审过程的记录包含在补充信息中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号