Cell Chemical Biology ( IF 6.6 ) Pub Date : 2020-07-28 , DOI: 10.1016/j.chembiol.2020.07.006 Urjita H Shah 1 , Rudy Toneatti 1 , Supriya A Gaitonde 1 , Jong M Shin 1 , Javier González-Maeso 1
|
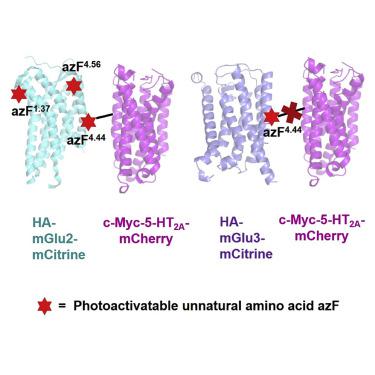
G protein-coupled receptors (GPCRs) are critical mediators of cell signaling. Although capable of activating G proteins in a monomeric form, numerous studies reveal a possible association of class A GPCRs into dimers/oligomers. The relative location of individual protomers within these GPCR complexes remains a topic of intense debate. We previously reported that class A serotonin 5-HT2A receptor (5-HT2AR) and class C metabotropic glutamate 2 receptor (mGluR2) are able to form a GPCR heterocomplex. By introducing the photoactivatable unnatural amino acid p-azido-L-phenylalanine (azF) at selected individual positions along the transmembrane (TM) segments of mGluR2, we delineate the residues that physically interact at the heteromeric interface of the 5-HT2AR-mGluR2 complex. We show that 5-HT2AR crosslinked with azF incorporated at the intracellular end of mGluR2's TM4, while no crosslinking was observed at other positions along TM1 and TM4. Together, these findings provide important insights into the structural arrangement of the 5-HT2AR-mGluR2 complex.
中文翻译:

基因编码的光交联剂的位点特异性结合可定位活细胞中 GPCR 复合物的异聚界面。
G 蛋白偶联受体 (GPCR) 是细胞信号传导的关键介质。尽管能够以单体形式激活 G 蛋白,但大量研究揭示了 A 类 GPCR 可能与二聚体/寡聚体相关联。这些 GPCR 复合物中单个原体的相对位置仍然是一个激烈争论的话题。我们之前报道过 A 类血清素 5-HT 2A受体 (5-HT 2A R) 和 C 类代谢型谷氨酸 2 受体 (mGluR2) 能够形成 GPCR 异源复合物。通过在 mGluR2 的跨膜 (TM) 区段的选定单个位置引入可光活化的非天然氨基酸对叠氮基-L-苯丙氨酸 (azF),我们描绘了在 5-HT 2A的异聚界面处物理相互作用的残基R-mGluR2 复合物。我们显示 5-HT 2AR 与结合在 mGluR2 的 TM4 细胞内端的azF交联,而在 TM1 和 TM4 的其他位置没有观察到交联。总之,这些发现为 5-HT 2A R-mGluR2 复合物的结构排列提供了重要的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号