Cell ( IF 45.5 ) Pub Date : 2020-07-28 , DOI: 10.1016/j.cell.2020.07.033 James Chen 1 , Brandon Malone 1 , Eliza Llewellyn 1 , Michael Grasso 2 , Patrick M M Shelton 2 , Paul Dominic B Olinares 3 , Kashyap Maruthi 4 , Edward T Eng 4 , Hasan Vatandaslar 5 , Brian T Chait 3 , Tarun M Kapoor 2 , Seth A Darst 1 , Elizabeth A Campbell 1
|
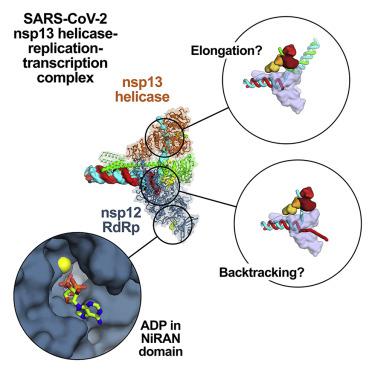
SARS-CoV-2 is the causative agent of the 2019–2020 pandemic. The SARS-CoV-2 genome is replicated and transcribed by the RNA-dependent RNA polymerase holoenzyme (subunits nsp7/nsp82/nsp12) along with a cast of accessory factors. One of these factors is the nsp13 helicase. Both the holo-RdRp and nsp13 are essential for viral replication and are targets for treating the disease COVID-19. Here we present cryoelectron microscopic structures of the SARS-CoV-2 holo-RdRp with an RNA template product in complex with two molecules of the nsp13 helicase. The Nidovirales order-specific N-terminal domains of each nsp13 interact with the N-terminal extension of each copy of nsp8. One nsp13 also contacts the nsp12 thumb. The structure places the nucleic acid-binding ATPase domains of the helicase directly in front of the replicating-transcribing holo-RdRp, constraining models for nsp13 function. We also observe ADP-Mg2+ bound in the nsp12 N-terminal nidovirus RdRp-associated nucleotidyltransferase domain, detailing a new pocket for anti-viral therapy development.
中文翻译:

SARS-CoV-2 复制-转录复合物中解旋酶-聚合酶偶联的结构基础。
SARS-CoV-2 is the causative agent of the 2019–2020 pandemic. The SARS-CoV-2 genome is replicated and transcribed by the RNA-dependent RNA polymerase holoenzyme (subunits nsp7/nsp82/nsp12) 以及一系列辅助因素。其中一个因素是 nsp13 解旋酶。holo-RdRp 和 nsp13 都是病毒复制所必需的,并且是治疗 COVID-19 疾病的靶点。在这里,我们展示了 SARS-CoV-2 holo-RdRp 的低温电子显微结构,其 RNA 模板产物与两个 nsp13 解旋酶分子复合。每个 nsp13 的 Nidovirales 顺序特定的 N 末端域与每个 nsp8 副本的 N 末端扩展相互作用。一个 nsp13 还与 nsp12 thumb 联系。该结构将解旋酶的核酸结合 ATPase 结构域直接置于复制-转录全息-RdRp 的前面,从而限制了 nsp13 功能的模型。我们还观察到 ADP-Mg 2+结合在 nsp12 N 末端巢状病毒 RdRp 相关核苷酸转移酶结构域中,详细说明了抗病毒治疗开发的新口袋。











































 京公网安备 11010802027423号
京公网安备 11010802027423号