Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics ( IF 2.5 ) Pub Date : 2020-07-28 , DOI: 10.1016/j.bbapap.2020.140512 David Flores-Solis 1 , Angeles Mendoza 1 , Itzel Rentería-González 2 , Luz E Casados-Vazquez 2 , Carlos H Trasviña-Arenas 2 , Pedro Jiménez-Sandoval 2 , Claudia G Benítez-Cardoza 3 , Federico Del Río-Portilla 1 , Luis G Brieba 2
|
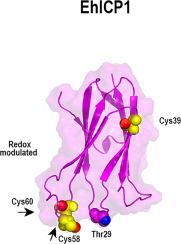
The genome of Entamoeba histolytica encodes approximately 50 Cysteine Proteases (CPs) whose activity is regulated by two Inhibitors of Cysteine Proteases (ICPs), EhICP1 and EhICP2. The main difference between both EhICPs is the acquisition of a 17 N-terminal targeting signal in EhICP2 and three exposed cysteine residues in EhICP1. The three exposed cysteines in EhICP1 potentiate the formation of cross-linking species that drive heterogeneity. Here we solved the NMR structure of EhICP1 using a mutant protein without accessible cysteines. Our structural data shows that EhICP1 adopts an immunoglobulin fold composed of seven β-strands, and three solvent exposed loops that resemble the structures of EhICP2 and chagasin. EhICP1 and EhICP2 are able to inhibit the archetypical cysteine protease papain by intercalating their BC loops into the protease active site independently of the character of the residue (serine or threonine) responsible to interact with the active site of papain. EhICP1 and EhICP2 present signals of functional divergence as they clustered in different clades. Two of the three exposed cysteines in EhICP1 are located at the DE loop that intercalates into the CP substrate-binding cleft. We propose that the solvent exposed cysteines of EhICP1 play a role in regulating its inhibitory activity and that in oxidative conditions, the cysteines of EhICP1 react to form intra and intermolecular disulfide bonds that render an inactive inhibitor. EhICP2 is not subject to redox regulation, as this inhibitor does not contain a single cysteine residue. This proposed redox regulation may be related to the differential cellular localization between EhICP1 and EhICP2.
中文翻译:

来自解组织变形虫的半胱氨酸蛋白酶1的抑制剂的溶液结构揭示了可能的自动调节机制。
溶血性变形虫的基因组编码大约50个半胱氨酸蛋白酶(CPs),其活性受两种半胱氨酸蛋白酶(ICPs)抑制剂EhICP1和EhICP2调节。两种EhICP之间的主要区别是在EhICP2中获得了17个N端靶向信号,在EhICP1中获得了三个暴露的半胱氨酸残基。EhICP1中的三个暴露的半胱氨酸增强了驱动异质性的交联物种的形成。在这里,我们使用没有可接近的半胱氨酸的突变蛋白解决了EhICP1的NMR结构。我们的结构数据表明,EhICP1采用了由七个β链和三个与EhICP2和查加辛类似的暴露于溶剂的环组成的免疫球蛋白折叠。EhICP1和EhICP2能够通过将BC环插入蛋白酶活性位点来抑制原型半胱氨酸蛋白酶木瓜蛋白酶,而与负责与木瓜蛋白酶活性位点相互作用的残基(丝氨酸或苏氨酸)的特征无关。当EhICP1和EhICP2聚集在不同进化枝中时,它们会呈现功能差异的信号。EhICP1中三个暴露的半胱氨酸中的两个位于插入到CP底物结合裂隙的DE环上。我们建议,暴露于EhICP1的半胱氨酸在调节其抑制活性中起作用,并且在氧化条件下,EhICP1的半胱氨酸起反应形成内部和分子间二硫键,从而使抑制剂失活。EhICP2不受氧化还原调节,因为该抑制剂不包含单个半胱氨酸残基。











































 京公网安备 11010802027423号
京公网安备 11010802027423号