Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease ( IF 4.2 ) Pub Date : 2020-07-28 , DOI: 10.1016/j.bbadis.2020.165905 Iris A L Silva 1 , Tereza Doušová 2 , Sofia Ramalho 1 , Raquel Centeio 1 , Luka A Clarke 1 , Violeta Railean 1 , Hugo M Botelho 1 , Andrea Holubová 3 , Iveta Valášková 4 , Jiunn-Tyng Yeh 5 , Tzyh-Chang Hwang 5 , Carlos M Farinha 1 , Karl Kunzelmann 6 , Margarida D Amaral 1
|
Background
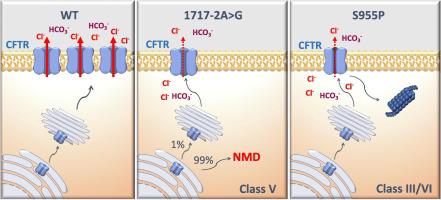
For most of the >2000 CFTR gene variants reported, neither the associated disease liability nor the underlying basic defect are known, and yet these are essential for disease prognosis and CFTR-based therapeutics. Here we aimed to characterize two ultra-rare mutations - 1717-2A > G (c.1585-2A > G) and S955P (p.Ser955Pro) - as case studies for personalized medicine.
Methods
Patient-derived rectal biopsies and intestinal organoids from two individuals with each of these mutations and F508del (p.Phe508del) in the other allele were used to assess CFTR function, response to modulators and RNA splicing pattern. In parallel, we used cellular models to further characterize S955P independently of F508del and to assess its response to CFTR modulators.
Results
Results in both rectal biopsies and intestinal organoids from both patients evidence residual CFTR function. Further characterization shows that 1717-2A > G leads to alternative splicing generating <1% normal CFTR mRNA and that S955P affects CFTR gating. Finally, studies in organoids predict that both patients are responders to VX-770 alone and even more to VX-770 combined with VX-809 or VX-661, although to different levels.
Conclusion
This study demonstrates the high potential of personalized medicine through theranostics to extend the label of approved drugs to patients with rare mutations.
中文翻译:

类器官作为治疗囊性纤维化超罕见突变的个性化医疗工具:S955P 和 1717-2A>G 的案例。
背景
对于大多数已报道的超过 2000 个 CFTR 基因变异,相关的疾病倾向和潜在的基本缺陷均未知,但这些对于疾病预后和基于 CFTR 的治疗至关重要。在这里,我们旨在描述两种极其罕见的突变 - 1717-2A > G (c.1585-2A > G) 和 S955P (p.Ser955Pro) - 作为个性化医疗的案例研究。
方法
使用来自两个具有这些突变和另一个等位基因中的 F508del (p.Phe508del) 的个体的患者来源的直肠活检和肠道类器官来评估 CFTR 功能、对调节剂的反应和 RNA 剪接模式。与此同时,我们使用细胞模型进一步表征独立于 F508del 的 S955P,并评估其对 CFTR 调节剂的反应。
结果
两名患者的直肠活检和肠道类器官结果均显示残余 CFTR 功能。进一步的表征表明,1717-2A > G 导致选择性剪接产生 <1% 正常 CFTR mRNA,并且 S955P 影响 CFTR 门控。最后,类器官研究预测,两名患者对单独使用 VX-770 均有反应,对 VX-770 联合 VX-809 或 VX-661 的反应甚至更高,尽管程度不同。
结论
这项研究证明了通过治疗诊断学进行个性化医疗的巨大潜力,可以将已批准药物的标签扩展到具有罕见突变的患者。











































 京公网安备 11010802027423号
京公网安备 11010802027423号