Biochimica et Biophysica Acta (BBA) - Bioenergetics ( IF 3.4 ) Pub Date : 2020-07-28 , DOI: 10.1016/j.bbabio.2020.148279 Souhela Boughanemi 1 , Pascale Infossi 1 , Marie-Thérèse Giudici-Orticoni 1 , Barbara Schoepp-Cothenet 1 , Marianne Guiral 1
|
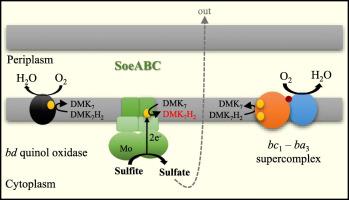
The microaerophilic bacterium Aquifex aeolicus is a chemolitoautotroph that uses sulfur compounds as electron sources. The model of oxidation of the energetic sulfur compounds in this bacterium predicts that sulfite would probably be a metabolic intermediate released in the cytoplasm. In this work, we purified and characterized a membrane-bound sulfite dehydrogenase, identified as an SoeABC enzyme, that was previously described as a sulfur reductase. It is a member of the DMSO-reductase family of molybdenum enzymes. This type of enzyme was identified a few years ago but never purified, and biochemical data and kinetic properties were completely lacking. An enzyme catalyzing sulfite oxidation using Nitro-blue tetrazolium as artificial electron acceptor was extracted from the membrane fraction of Aquifex aeolicus. The purified enzyme is a dimer of trimer (αβγ)2 of about 390 kDa. The KM for sulfite and kcat values were 34 μM and 567 s−1 respectively, at pH 8.3 and 55 °C. We furthermore showed that SoeABC reduces a UQ10 analogue, the decyl-ubiquinone, as well, with a KM of 2.6 μM and a kcat of 52.9 s−1. It seems to specifically oxidize sulfite but can work in the reverse direction, reduction of sulfur or tetrathionate, using reduced methyl viologen as electron donor. The close phylogenetic relationship of Soe with sulfur and tetrathionate reductases that we established, perfectly explains this enzymatic ability, although its bidirectionality in vivo still needs to be clarified. Oxygen-consumption measurements confirmed that electrons generated by sulfite oxidation in the cytoplasm enter the respiratory chain at the level of quinones.
中文翻译:

来自细菌Aquifex aeolicus的还原醌的亚硫酸钼脱氢酶SoeABC氧化亚硫酸盐。
微需氧细菌Aquifex aeolicus是一种化学自养生物,使用硫化合物作为电子源。该细菌中高能硫化合物的氧化模型预测,亚硫酸盐可能是细胞质中释放的代谢中间体。在这项工作中,我们纯化并鉴定了膜结合的亚硫酸盐脱氢酶,被鉴定为SoeABC酶,以前称为硫还原酶。它是DMSO钼酶还原酶家族的成员。这种酶在几年前被发现,但从未纯化过,并且完全缺乏生化数据和动力学特性。从膜的膜中提取了以硝基蓝四氮作为人工电子受体的催化亚硫酸盐氧化的酶。Aquifex aeolicus。纯化的酶是约390kDa的三聚体(αβγ)2的二聚体。在pH 8.3和55°C下,亚硫酸盐的K M和k cat值分别为34μM和567 s -1。我们还表明,SoeABC还可以还原UQ 10类似物癸基-泛醌,其K M为2.6μM,k cat为52.9 s -1。它似乎能特异性氧化亚硫酸盐,但可以使用还原的甲基紫精作为电子供体,以相反的方向进行反应,还原出硫或四硫代硫酸盐。尽管我们仍需要弄清其在体内的双向性,但我们建立的Soe与硫和四硫酸盐还原酶的密切系统发育关系完美地解释了这种酶促能力。耗氧量测量证实,亚硫酸盐在细胞质中氧化产生的电子以醌的水平进入呼吸链。











































 京公网安备 11010802027423号
京公网安备 11010802027423号