当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
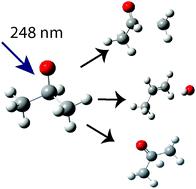
Photodissociation of iso-propoxy (i-C3H7O) radical at 248 nm.
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2020-07-27 , DOI: 10.1039/d0cp02493g Erin N Sullivan 1 , Steven Saric , Daniel M Neumark
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2020-07-27 , DOI: 10.1039/d0cp02493g Erin N Sullivan 1 , Steven Saric , Daniel M Neumark
Affiliation
|
Photodissociation of the i-C3H7O radical is investigated using fast beam photofragment translational spectroscopy. Neutral i-C3H7O radicals are produced through the photodetachment of a fast beam of i-C3H7O− anions and are subsequently dissociated at 248 nm (5.0 eV). The dominant product channels are CH3 + CH3CHO and OH + C3H6 with some contribution from H + C3H6O. The CH3 and H loss channels are attributed to dissociation on the ground electronic state of i-C3H7O, but in a nonstatistical manner because calculated RRKM dissociation rates exceed the rate of energy randomization. Translational energy and angular distributions for OH loss are consistent with ground state dissociation, but the branching ratio for this channel is considerably higher than predicted from RRKM rate calculations. Additionally, i-C3H7O undergoes three-body fragmentation to CH3 + CH3 + HCO and CH3 + CH4 + CO. These three-body channels are attributed to dissociation of i-C3H7O to CH3 + CH3CHO, followed by secondary dissociation of CH3CHO on its ground electronic state.
中文翻译:

异丙氧基(i-C3H7O)自由基在248 nm处的光解离。
iC 3 H 7 O自由基的光解离使用快速光束片段平移光谱学进行了研究。中性IC 3 ħ 7个O自由基通过IC的快速束的光致产生的3 ħ 7 ö -阴离子和在248nm(5.0电子伏特),随后解离。占主导地位的产物通道是CH 3 + CH 3 CHO和OH + C 3 H ^ 6与选自H + C一些贡献3 ħ 6 O的CH 3和H损失信道被归因于解离于IC的基电子态3H 7 O,但采用非统计方式,因为计算出的RRKM解离速率超过了能量随机化速率。OH损失的平移能和角分布与基态解离一致,但是该通道的分支比大大高于RRKM速率计算所预测的比率。此外,iC 3 H 7 O经历三体断裂,成为CH 3 + CH 3 + HCO和CH 3 + CH 4 + CO。这些三体通道归因于iC 3 H 7 O分解为CH 3 + CH 3 CHO,然后进行CH的二级解离3 CHO处于其基础电子状态。
更新日期:2020-08-18
中文翻译:

异丙氧基(i-C3H7O)自由基在248 nm处的光解离。
iC 3 H 7 O自由基的光解离使用快速光束片段平移光谱学进行了研究。中性IC 3 ħ 7个O自由基通过IC的快速束的光致产生的3 ħ 7 ö -阴离子和在248nm(5.0电子伏特),随后解离。占主导地位的产物通道是CH 3 + CH 3 CHO和OH + C 3 H ^ 6与选自H + C一些贡献3 ħ 6 O的CH 3和H损失信道被归因于解离于IC的基电子态3H 7 O,但采用非统计方式,因为计算出的RRKM解离速率超过了能量随机化速率。OH损失的平移能和角分布与基态解离一致,但是该通道的分支比大大高于RRKM速率计算所预测的比率。此外,iC 3 H 7 O经历三体断裂,成为CH 3 + CH 3 + HCO和CH 3 + CH 4 + CO。这些三体通道归因于iC 3 H 7 O分解为CH 3 + CH 3 CHO,然后进行CH的二级解离3 CHO处于其基础电子状态。



























 京公网安备 11010802027423号
京公网安备 11010802027423号