当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diazaphosphinyl radical-catalyzed deoxygenation of α-carboxy ketones: a new protocol for chemo-selective C–O bond scission via mechanism regulation
Chemical Science ( IF 7.6 ) Pub Date : 2020-07-27 , DOI: 10.1039/d0sc03220d Jingjing Zhang 1 , Jin-Dong Yang 1 , Jin-Pei Cheng 1, 2
Chemical Science ( IF 7.6 ) Pub Date : 2020-07-27 , DOI: 10.1039/d0sc03220d Jingjing Zhang 1 , Jin-Dong Yang 1 , Jin-Pei Cheng 1, 2
Affiliation
|
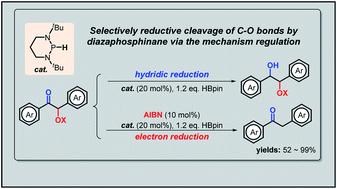
C–O bond cleavage is often a key process in defunctionalization of organic compounds as well as in degradation of natural polymers. However, it seldom occurs regioselectively for different types of C–O bonds under metal-free mild conditions. Here we report a facile chemo-selective cleavage of the α-C–O bonds in α-carboxy ketones by commercially available pinacolborane under the catalysis of diazaphosphinane based on a mechanism switch strategy. This new reaction features high efficiency, low cost and good group-tolerance, and is also amenable to catalytic deprotection of desyl-protected carboxylic acids and amino acids. Mechanistic studies indicated an electron-transfer-initiated radical process, underlining two crucial steps: (1) the initiator azodiisobutyronitrile switches originally hydridic reduction to kinetically more accessible electron reduction; and (2) the catalytic phosphorus species upconverts weakly reducing pinacolborane into strongly reducing diazaphosphinane.
中文翻译:

二氮杂膦基自由基催化α-羧基酮脱氧:通过机制调节化学选择性C-O键断裂的新方案
C-O键断裂通常是有机化合物去官能化以及天然聚合物降解的关键过程。然而,在无金属的温和条件下,不同类型的C-O键很少发生区域选择性。在这里,我们报告了基于机制转换策略,在二氮杂膦烷的催化下,通过市售的频那醇硼烷对α-羧基酮中的α-C-O键进行化学选择性裂解。这种新反应具有效率高、成本低和良好的基团耐受性的特点,并且还适用于去酰基保护的羧酸和氨基酸的催化脱保护。机理研究表明电子转移引发的自由基过程,强调了两个关键步骤:(1)引发剂偶氮二异丁腈将最初的氢还原转变为动力学上更容易进行的电子还原; (2)催化磷物质将弱还原性频哪醇硼烷上转换为强还原性二氮杂膦烷。
更新日期:2020-08-20
中文翻译:

二氮杂膦基自由基催化α-羧基酮脱氧:通过机制调节化学选择性C-O键断裂的新方案
C-O键断裂通常是有机化合物去官能化以及天然聚合物降解的关键过程。然而,在无金属的温和条件下,不同类型的C-O键很少发生区域选择性。在这里,我们报告了基于机制转换策略,在二氮杂膦烷的催化下,通过市售的频那醇硼烷对α-羧基酮中的α-C-O键进行化学选择性裂解。这种新反应具有效率高、成本低和良好的基团耐受性的特点,并且还适用于去酰基保护的羧酸和氨基酸的催化脱保护。机理研究表明电子转移引发的自由基过程,强调了两个关键步骤:(1)引发剂偶氮二异丁腈将最初的氢还原转变为动力学上更容易进行的电子还原; (2)催化磷物质将弱还原性频哪醇硼烷上转换为强还原性二氮杂膦烷。











































 京公网安备 11010802027423号
京公网安备 11010802027423号