Journal of Chromatography B ( IF 3 ) Pub Date : 2020-07-27 , DOI: 10.1016/j.jchromb.2020.122283 Abhishek Gour 1 , Ashish Dogra 1 , Priya Wazir 2 , Gurdarshan Singh 1 , Utpal Nandi 1
|
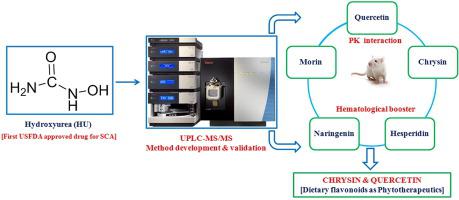
Hydroxyurea (HU) is the first-ever approved drug by the United States Food and Drug Administration (USFDA) for the management of sickle cell anemia (SCA). However, its treatment is associated with severe liabilities like myelosuppression. Therefore, the aim of the present investigation was to identify phytotherapeutics through assessment of the pharmacokinetic interaction of HU with dietary bioflavonoid followed by elucidation of the same phytoconstituent to protect HU-induced toxicity in hematological profile. In this direction, we developed a sensitive ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) method to estimate HU in rat plasma at first and then validated as per USFDA guidelines as there is no such precedent in the literature. A simple plasma protein precipitation method was employed for plasma sample processing. The separation was achieved in gradient mode using Syncronis HILIC column (100 × 4.6 mm, 3 μm) with a mobile phase composition of water containing 0.1% (v/v) formic acid and acetonitrile. Ionization was carried out in positive heated-electrospray ionization (H-ESI) mode. Detection was done in selected reaction monitoring (SRM) mode with m/z 77.1 > 44.4 and m/z 75.1 > 58.2 for HU and methylurea (internal standard), respectively. All the validation parameters were within the acceptable criteria. This bioanalytical method was found to be useful in assessing the preclinical pharmacokinetic interaction of HU. Concomitant administration of chrysin or quercetin with HU in rats significantly enhanced the oral exposure of HU. Lowering of total red blood cells (RBC) and hemoglobin (Hb) level by HU in rats was significantly improved in the presence of chrysin, quercetin, and naringenin. Overall, both chrysin and quercetin showed potential to be a promising phytotherapeutics for concomitant therapy with HU to combat its dose-dependent side effects.
中文翻译:

一种用于羟基脲的高度灵敏的UPLC-MS / MS方法,用于评估植物治疗剂对大鼠的药代动力学干预。
羟基脲(HU)是美国食品和药物管理局(USFDA)首次批准的用于治疗镰状细胞性贫血(SCA)的药物。但是,其治疗与严重的症状如骨髓抑制有关。因此,本研究的目的是通过评估HU与膳食生物类黄酮的药代动力学相互作用来鉴定植物治疗药,然后阐明相同的植物成分以保护HU引起的血液学毒性。为此,我们开发了一种灵敏的超高效液相色谱-串联质谱(UPLC-MS / MS)方法来首先估算大鼠血浆中的HU,然后根据USFDA指南进行验证,因为在文献中尚无此类先例。一种简单的血浆蛋白沉淀方法用于血浆样品处理。使用Syncronis HILIC色谱柱(100×4.6 mm,3μm)以流动相组成的水(含0.1%(v / v)甲酸和乙腈)以梯度模式实现分离。电离以正加热电喷雾电离(H-ESI)模式进行。在选定的反应监测(SRM)模式下分别对HU和甲基脲(内标)进行m / z 77.1> 44.4和m / z 75.1> 58.2的检测。所有验证参数均在可接受的标准之内。发现这种生物分析方法可用于评估HU的临床前药代动力学相互作用。在大鼠中并发黄ry或槲皮素与HU一起显着增强了HU的口服暴露。在存在chrysin,槲皮素和naringenin的情况下,HU降低了大鼠总红细胞(RBC)和血红蛋白(Hb)的水平,并得到了明显改善。总体而言,对于与HU伴随治疗以对抗其剂量依赖性副作用的问题,Chrysin和quercetin均显示出有望成为一种有前途的植物治疗药。



























 京公网安备 11010802027423号
京公网安备 11010802027423号