当前位置:
X-MOL 学术
›
Solid State Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Comparative evaluation of copper oxide nano-photocatalyst characteristics by formation of composite with TiO2 and ZnO
Solid State Sciences ( IF 3.4 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.solidstatesciences.2020.106362 K. Sabzehei , S.H. Hadavi , M. Gharibiyan Bajestani , S. Sheibani
Solid State Sciences ( IF 3.4 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.solidstatesciences.2020.106362 K. Sabzehei , S.H. Hadavi , M. Gharibiyan Bajestani , S. Sheibani
|
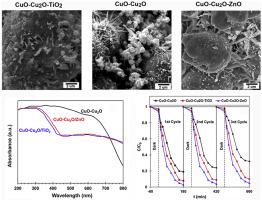
Abstract In this paper, the effect of composite formation of CuO–Cu2O with TiO2 and ZnO on photocatalytic degradation of methylene blue (MB) dye was studied under visible light irradiation. The X-ray diffraction (XRD) and Raman results revealed that both Cu2O and CuO phases were obtained. Field emission scanning electron microscopy (FESEM) results showed the uniform coverage of copper oxides with TiO2 and ZnO nanoparticles which will be beneficial for charge carriers transform between different phases and copper oxides. The highly decreased photoluminescence (PL) intensity of CuO–Cu2O–ZnO photocatalyst indicated better charge separation compared to those of other samples. However, diffuse reflectance spectroscopy (DRS) results showed the increased band gap energy from 1.6 to about 2.6 and 2.7 eV by the addition of ZnO and TiO2 over CuO–Cu2O, respectively. Relatively complete degradation of 5 mg L−1 of MB solution was obtained by CuO–Cu2O–ZnO photocatalyst after 180 min visible light irradiation. A study on the kinetics and mechanism of the photodegradation process showed the rate-determining step was the formation of hydroxyl radicals. The activation energy for photodegradation of MB by CuO–Cu2O was 17.7 kJ mol−1, which can be reduced to 8.8 kJ mol−1 by adding ZnO. Study on the thermodynamics of photocatalysis in the temperature range of 288–318 K using the activated complex theory revealed the endothermic and nonspontaneous features of the photocatalytic process.
中文翻译:

TiO2和ZnO复合形成氧化铜纳米光催化剂特性的比较评价
摘要 本文研究了可见光照射下CuO-Cu2O与TiO2和ZnO复合形成对亚甲基蓝(MB)染料光催化降解的影响。X 射线衍射 (XRD) 和拉曼结果表明获得了 Cu2O 和 CuO 相。场发射扫描电子显微镜 (FESEM) 结果显示氧化铜与 TiO2 和 ZnO 纳米颗粒的均匀覆盖,这将有利于不同相和氧化铜之间的电荷载流子转换。与其他样品相比,CuO-Cu2O-ZnO 光催化剂显着降低的光致发光(PL)强度表明电荷分离更好。然而,漫反射光谱 (DRS) 结果表明,通过在 CuO-Cu2O 上添加 ZnO 和 TiO2,带隙能量从 1.6 eV 增加到约 2.6 eV 和 2.7 eV,分别。在可见光照射 180 分钟后,CuO-Cu2O-ZnO 光催化剂可相对完全降解 5 mg L-1 的 MB 溶液。对光降解过程的动力学和机制的研究表明,决定速率的步骤是羟基自由基的形成。CuO-Cu2O 光降解 MB 的活化能为 17.7 kJ mol-1,添加 ZnO 可将其降低至 8.8 kJ mol-1。使用活化络合物理论在 288-318 K 温度范围内研究光催化的热力学揭示了光催化过程的吸热和非自发特征。对光降解过程的动力学和机制的研究表明,决定速率的步骤是羟基自由基的形成。CuO-Cu2O 光降解 MB 的活化能为 17.7 kJ mol-1,添加 ZnO 可将其降低至 8.8 kJ mol-1。使用活化络合物理论在 288-318 K 温度范围内研究光催化的热力学揭示了光催化过程的吸热和非自发特征。对光降解过程动力学和机制的研究表明,决定速率的步骤是羟基自由基的形成。CuO-Cu2O 光降解 MB 的活化能为 17.7 kJ mol-1,添加 ZnO 可将其降低至 8.8 kJ mol-1。使用活化络合物理论在 288-318 K 温度范围内研究光催化的热力学揭示了光催化过程的吸热和非自发特征。
更新日期:2020-09-01
中文翻译:

TiO2和ZnO复合形成氧化铜纳米光催化剂特性的比较评价
摘要 本文研究了可见光照射下CuO-Cu2O与TiO2和ZnO复合形成对亚甲基蓝(MB)染料光催化降解的影响。X 射线衍射 (XRD) 和拉曼结果表明获得了 Cu2O 和 CuO 相。场发射扫描电子显微镜 (FESEM) 结果显示氧化铜与 TiO2 和 ZnO 纳米颗粒的均匀覆盖,这将有利于不同相和氧化铜之间的电荷载流子转换。与其他样品相比,CuO-Cu2O-ZnO 光催化剂显着降低的光致发光(PL)强度表明电荷分离更好。然而,漫反射光谱 (DRS) 结果表明,通过在 CuO-Cu2O 上添加 ZnO 和 TiO2,带隙能量从 1.6 eV 增加到约 2.6 eV 和 2.7 eV,分别。在可见光照射 180 分钟后,CuO-Cu2O-ZnO 光催化剂可相对完全降解 5 mg L-1 的 MB 溶液。对光降解过程的动力学和机制的研究表明,决定速率的步骤是羟基自由基的形成。CuO-Cu2O 光降解 MB 的活化能为 17.7 kJ mol-1,添加 ZnO 可将其降低至 8.8 kJ mol-1。使用活化络合物理论在 288-318 K 温度范围内研究光催化的热力学揭示了光催化过程的吸热和非自发特征。对光降解过程的动力学和机制的研究表明,决定速率的步骤是羟基自由基的形成。CuO-Cu2O 光降解 MB 的活化能为 17.7 kJ mol-1,添加 ZnO 可将其降低至 8.8 kJ mol-1。使用活化络合物理论在 288-318 K 温度范围内研究光催化的热力学揭示了光催化过程的吸热和非自发特征。对光降解过程动力学和机制的研究表明,决定速率的步骤是羟基自由基的形成。CuO-Cu2O 光降解 MB 的活化能为 17.7 kJ mol-1,添加 ZnO 可将其降低至 8.8 kJ mol-1。使用活化络合物理论在 288-318 K 温度范围内研究光催化的热力学揭示了光催化过程的吸热和非自发特征。











































 京公网安备 11010802027423号
京公网安备 11010802027423号