当前位置:
X-MOL 学术
›
Fluid Phase Equilibr.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Equilibrium CO2 solubility in the aqueous mixture of MAE and AEEA: Experimental study and development of modified thermodynamic model
Fluid Phase Equilibria ( IF 2.8 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.fluid.2020.112766 Diwakar Pandey , Monoj Kumar Mondal
Fluid Phase Equilibria ( IF 2.8 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.fluid.2020.112766 Diwakar Pandey , Monoj Kumar Mondal
|
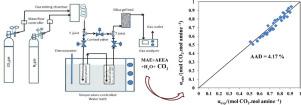
Abstract In the present study, equilibrium solubility of CO2 was studied in the aqueous blend of 2-(methylamino)ethanol (MAE) and aminoethylethanolamine (AEEA). Total concentration of blend solution, weight fraction of AEEA in aqueous (MAE+AEEA) blend, temperature, and partial pressure of CO2 was varied from 10 to 30 wt %, 0.10 to 0.30, 298.15 to 323.15 K, and 8.11 to 20.67 kPa, respectively. Maximum loading 0.944 mol CO2/mol amine was occurred at 298.15 K, 20.27 kPa of CO2 partial pressure, 10 wt % total concentration with 0.30 wt fraction AEEA. The Kent-Eisenberg thermodynamic model was modified by incorporating newly introduced correction factor (Fk) to predict CO2 solubility in the aqueous blend of MAE+AEEA. Experimental data and model predicted data was in good agreement with each other. In addition, heat of CO2 absorption was also calculated for this amine blend and reported as -73.43 kJ/mol.
中文翻译:

平衡 CO2 在 MAE 和 AEEA 的水性混合物中的溶解度:实验研究和改进的热力学模型的发展
摘要 在本研究中,研究了 CO2 在 2-(甲基氨基)乙醇 (MAE) 和氨基乙基乙醇胺 (AEEA) 的水性混合物中的平衡溶解度。共混溶液的总浓度、含水 (MAE+AEEA) 共混物中 AEEA 的重量分数、温度和 CO2 分压从 10 到 30 重量%、0.10 到 0.30、298.15 到 323.15 K 和 8.11 到 20.67 kPa 不等,分别。最大负载 0.944 mol CO2/mol 胺发生在 298.15 K、20.27 kPa CO2 分压、10 wt% 总浓度和 0.30 wt 份 AEEA 时。Kent-Eisenberg 热力学模型通过引入新引入的校正因子 (Fk) 进行修改,以预测 MAE+AEEA 的水性混合物中的 CO2 溶解度。实验数据与模型预测数据吻合良好。此外,
更新日期:2020-11-01
中文翻译:

平衡 CO2 在 MAE 和 AEEA 的水性混合物中的溶解度:实验研究和改进的热力学模型的发展
摘要 在本研究中,研究了 CO2 在 2-(甲基氨基)乙醇 (MAE) 和氨基乙基乙醇胺 (AEEA) 的水性混合物中的平衡溶解度。共混溶液的总浓度、含水 (MAE+AEEA) 共混物中 AEEA 的重量分数、温度和 CO2 分压从 10 到 30 重量%、0.10 到 0.30、298.15 到 323.15 K 和 8.11 到 20.67 kPa 不等,分别。最大负载 0.944 mol CO2/mol 胺发生在 298.15 K、20.27 kPa CO2 分压、10 wt% 总浓度和 0.30 wt 份 AEEA 时。Kent-Eisenberg 热力学模型通过引入新引入的校正因子 (Fk) 进行修改,以预测 MAE+AEEA 的水性混合物中的 CO2 溶解度。实验数据与模型预测数据吻合良好。此外,











































 京公网安备 11010802027423号
京公网安备 11010802027423号