当前位置:
X-MOL 学术
›
Fluid Phase Equilibr.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility of carbon dioxide (CO2) in four bis (Trifluoromethyl-Sulfonyl) imide ([Tf2N]) based ionic liquids
Fluid Phase Equilibria ( IF 2.8 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.fluid.2020.112757 Devjyoti Nath , Amr Henni
Fluid Phase Equilibria ( IF 2.8 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.fluid.2020.112757 Devjyoti Nath , Amr Henni
|
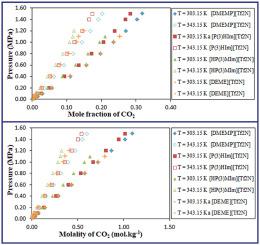
Abstract The solubility of carbon dioxide (CO2) in four bis(trifluoromethylsulfonyl)imide ([Tf2N]) based ionic liquids was investigated in this study. The solubility of CO2 in four ionic liquids {N,n-dimethyl-n-ethyl-n-(3-methoxypropyl)ammonium bis(trifluoromethylsulfonyl)imide ([DMEMP][Tf2N]), 1-Allyl-3H-imidazolium bis(trifluoromethylsulfonyl)imide ([P(3)HIm][Tf2N]), 1-(3-Hydroxypropyl)-3-methylimidazolium bis(trifluoromethyl-sulfonyl)imide ([HP(3)MIm][Tf2N]) and N,n-diethyl-n-methyl-n(2-methoxyethyl)ammonium bis(trifluoromethanesulfonyl)imide ([DEME][Tf2N])} was measured at (303.15, 323.15, and 343.15) K and at pressures up to 1.5 MPa using a gravimetric microbalance. The densities of these ionic liquids (ILs) were also measured at a temperatures range varying from (293.15 K–343.15 K) at atmospheric pressure (95.1 kPa with 0.8 kPa uncertainty). The experimental solubility data were correlated with the Peng-Robinson (PR) equation of state using three mixing rules (i) van der Waals one (vdW1), (ii) van der Waals two (vdW2), and (iii) Wong-Sandler mixing rules combined with NRTL model (WS-NRTL). Henry's law constants for the absorption of CO2 in these ionic liquids were estimated from the solubility data, and the enthalpy and entropy for the absorption of CO2 in these ILs at infinite dilution were also calculated from the estimated Henry's law constants. Results show that [DMEMP][Tf2N] has one of the highest capacities for CO2 compared to other ionic liquids of similar sizes in the literature.
中文翻译:

二氧化碳 (CO2) 在四种双(三氟甲基磺酰基)酰亚胺 ([Tf2N]) 离子液体中的溶解度
摘要 本研究研究了二氧化碳 (CO2) 在四种双(三氟甲基磺酰基)酰亚胺 ([Tf2N]) 基离子液体中的溶解度。CO2在四种离子液体中的溶解度{N,n-二甲基-n-乙基-n-(3-甲氧基丙基)铵双(三氟甲基磺酰基)亚胺([DMEMP][Tf2N])、1-烯丙基-3H-咪唑鎓双(三氟甲基磺酰基)酰亚胺 ([P(3)HIm][Tf2N])、1-(3-羟丙基)-3-甲基咪唑鎓双(三氟甲基-磺酰基)酰亚胺 ([HP(3)MIm][Tf2N]) 和 N,n -二乙基-n-甲基-n(2-甲氧基乙基)铵双(三氟甲磺酰基)亚胺([DEME][Tf2N])}在(303.15、323.15和343.15)K和高达1.5 MPa的压力下使用重量分析法进行测量微量天平。这些离子液体 (IL) 的密度也在大气压力(95.1 kPa,不确定性为 0.8 kPa)下(293.15 K–343.15 K)的温度范围内测量。使用三种混合规则 (i) 范德华力一 (vdW1),(ii) 范德华力二 (vdW2) 和 (iii) Wong-Sandler 将实验溶解度数据与 Peng-Robinson (PR) 状态方程相关联混合规则结合 NRTL 模型 (WS-NRTL)。这些离子液体中 CO2 吸收的亨利定律常数是根据溶解度数据估算的,并且这些离子液体在无限稀释时吸收 CO2 的焓和熵也由估算的亨利定律常数计算得出。结果表明,与文献中类似尺寸的其他离子液体相比,[DMEMP][Tf2N] 具有最高的 CO2 容量之一。(iii) Wong-Sandler 混合规则结合 NRTL 模型 (WS-NRTL)。这些离子液体中 CO2 吸收的亨利定律常数是根据溶解度数据估算的,并且这些离子液体在无限稀释时吸收 CO2 的焓和熵也由估算的亨利定律常数计算得出。结果表明,与文献中类似尺寸的其他离子液体相比,[DMEMP][Tf2N] 具有最高的 CO2 容量之一。(iii) Wong-Sandler 混合规则结合 NRTL 模型 (WS-NRTL)。这些离子液体中 CO2 吸收的亨利定律常数是根据溶解度数据估算的,并且这些离子液体在无限稀释时吸收 CO2 的焓和熵也由估算的亨利定律常数计算得出。结果表明,与文献中类似尺寸的其他离子液体相比,[DMEMP][Tf2N] 具有最高的 CO2 容量之一。
更新日期:2020-12-01
中文翻译:

二氧化碳 (CO2) 在四种双(三氟甲基磺酰基)酰亚胺 ([Tf2N]) 离子液体中的溶解度
摘要 本研究研究了二氧化碳 (CO2) 在四种双(三氟甲基磺酰基)酰亚胺 ([Tf2N]) 基离子液体中的溶解度。CO2在四种离子液体中的溶解度{N,n-二甲基-n-乙基-n-(3-甲氧基丙基)铵双(三氟甲基磺酰基)亚胺([DMEMP][Tf2N])、1-烯丙基-3H-咪唑鎓双(三氟甲基磺酰基)酰亚胺 ([P(3)HIm][Tf2N])、1-(3-羟丙基)-3-甲基咪唑鎓双(三氟甲基-磺酰基)酰亚胺 ([HP(3)MIm][Tf2N]) 和 N,n -二乙基-n-甲基-n(2-甲氧基乙基)铵双(三氟甲磺酰基)亚胺([DEME][Tf2N])}在(303.15、323.15和343.15)K和高达1.5 MPa的压力下使用重量分析法进行测量微量天平。这些离子液体 (IL) 的密度也在大气压力(95.1 kPa,不确定性为 0.8 kPa)下(293.15 K–343.15 K)的温度范围内测量。使用三种混合规则 (i) 范德华力一 (vdW1),(ii) 范德华力二 (vdW2) 和 (iii) Wong-Sandler 将实验溶解度数据与 Peng-Robinson (PR) 状态方程相关联混合规则结合 NRTL 模型 (WS-NRTL)。这些离子液体中 CO2 吸收的亨利定律常数是根据溶解度数据估算的,并且这些离子液体在无限稀释时吸收 CO2 的焓和熵也由估算的亨利定律常数计算得出。结果表明,与文献中类似尺寸的其他离子液体相比,[DMEMP][Tf2N] 具有最高的 CO2 容量之一。(iii) Wong-Sandler 混合规则结合 NRTL 模型 (WS-NRTL)。这些离子液体中 CO2 吸收的亨利定律常数是根据溶解度数据估算的,并且这些离子液体在无限稀释时吸收 CO2 的焓和熵也由估算的亨利定律常数计算得出。结果表明,与文献中类似尺寸的其他离子液体相比,[DMEMP][Tf2N] 具有最高的 CO2 容量之一。(iii) Wong-Sandler 混合规则结合 NRTL 模型 (WS-NRTL)。这些离子液体中 CO2 吸收的亨利定律常数是根据溶解度数据估算的,并且这些离子液体在无限稀释时吸收 CO2 的焓和熵也由估算的亨利定律常数计算得出。结果表明,与文献中类似尺寸的其他离子液体相比,[DMEMP][Tf2N] 具有最高的 CO2 容量之一。











































 京公网安备 11010802027423号
京公网安备 11010802027423号