当前位置:
X-MOL 学术
›
J. Food Biochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structure characteristics of flavonoids for heterocyclic aromatic amines inhibition using quantitative structure-activity relationship modeling.
Journal of Food Biochemistry ( IF 4 ) Pub Date : 2020-07-25 , DOI: 10.1111/jfbc.13390 Lei Zhao 1 , Fei Pan 1 , Yubin Li 1 , Shuai Hao 1 , Arshad Mehmood 1 , Yong Wang 2 , Chengtao Wang 1
Journal of Food Biochemistry ( IF 4 ) Pub Date : 2020-07-25 , DOI: 10.1111/jfbc.13390 Lei Zhao 1 , Fei Pan 1 , Yubin Li 1 , Shuai Hao 1 , Arshad Mehmood 1 , Yong Wang 2 , Chengtao Wang 1
Affiliation
|
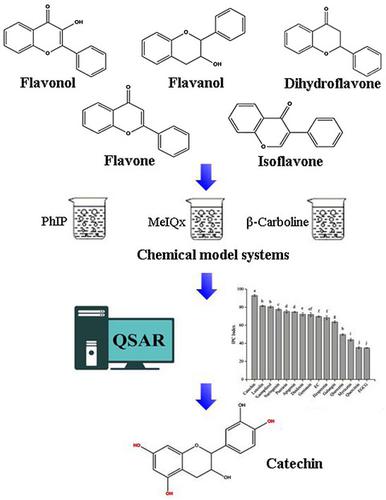
The objective of this study was to investigate the structure characteristics of flavonoids that act as inhibitors for heterocyclic aromatic amines (HAAs) formation. Five quantitative structure–activity relationship models for predicting the inhibitory rates of HAAs (norharman, harman, PhIP, MeIQx, and 4,8‐DiMeIQx) were established using selected chemometric parameters (R2: 0.591–0.920), and indicated that the hydrophobicity, hydroxyl groups, and topological structure of flavonoids played important roles in the inhibition of HAAs formation. The 5,7‐dihydroxyls in meta‐position of the A‐ring and the 4′‐hydroxyl in the B‐ring of flavonoids were critical for the inhibitory effects of HAAs, whereas the introduction of 3‐hydroxyl and 3‐O‐glucoside in the C‐ring reduced the inhibitory effects. Catechin served as the most effective inhibitor of HAAs followed by luteolin and genistein. The study can bring us a broader idea for controlling the formation of HAAs according to the structure of flavonoids.
中文翻译:

黄酮类化合物的结构特征用于杂环芳香胺的抑制作用,采用定量构效关系模型。
这项研究的目的是研究类黄酮的结构特征,它们可作为杂环芳香胺(HAAs)形成的抑制剂。使用选定的化学计量学参数(R 2:0.591–0.920)建立了五个定量的结构-活性关系模型,用于预测HAA的抑制率(norharman,harman,PhIP,MeIQx和4,8-DiMeIQx)。类黄酮的羟基,羟基和拓扑结构在抑制HAAs形成中起重要作用。类黄酮的A环和B环的4'-羟基之间的5,7-二羟基对于HAAs的抑制作用至关重要,而引入3-羟基和3- OC环中的葡糖苷减少了抑制作用。儿茶素是HAA最有效的抑制剂,其次是木犀草素和染料木黄酮。这项研究可以为我们带来更广泛的想法,可根据类黄酮的结构控制HAAs的形成。
更新日期:2020-09-14
中文翻译:

黄酮类化合物的结构特征用于杂环芳香胺的抑制作用,采用定量构效关系模型。
这项研究的目的是研究类黄酮的结构特征,它们可作为杂环芳香胺(HAAs)形成的抑制剂。使用选定的化学计量学参数(R 2:0.591–0.920)建立了五个定量的结构-活性关系模型,用于预测HAA的抑制率(norharman,harman,PhIP,MeIQx和4,8-DiMeIQx)。类黄酮的羟基,羟基和拓扑结构在抑制HAAs形成中起重要作用。类黄酮的A环和B环的4'-羟基之间的5,7-二羟基对于HAAs的抑制作用至关重要,而引入3-羟基和3- OC环中的葡糖苷减少了抑制作用。儿茶素是HAA最有效的抑制剂,其次是木犀草素和染料木黄酮。这项研究可以为我们带来更广泛的想法,可根据类黄酮的结构控制HAAs的形成。



























 京公网安备 11010802027423号
京公网安备 11010802027423号