Tetrahedron ( IF 2.1 ) Pub Date : 2020-07-25 , DOI: 10.1016/j.tet.2020.131436 Zheng Li , Ting-Ting Feng , Ying Zhou , You-Ping Tian , Wei Zhou , Xiong-Li Liu
|
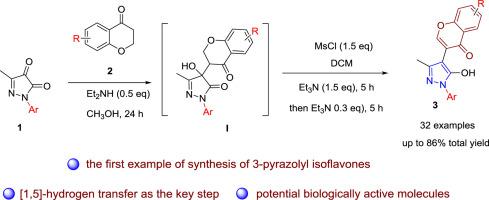
Inspired by the chemistry and biology of isoflavones and pyrazolones, herein we report the first example of synthesis of 3-pyrazolyl isoflavones by two steps, starting from commercially available chromanones and pyrazolones. The [1,5]-proton transfer was used as a key strategy. All the 3-pyrazolyl isoflavones 3, which are hard to synthesize by traditional methods, are smoothly obtained in up to 86% total yield. Interestingly, the product forms a stable 7-membered ring through intramolecular hydrogen bond between carbonyl group on chromone core and hydroxyl group on pyrazolone core, preventing further ketone-enol tautomerization. In particular, the products are featured with an intriguing combination of two privileged motifs including isoflavone and pyrazolone substructures, which might be valuable in medicinal chemistry.
中文翻译:

[1,5]-质子转移作为关键策略:迅速获得灵感源自天然产物的3-吡唑基异黄酮文库
受异黄酮和吡唑啉酮的化学和生物学的启发,在此,我们报道了从市售发色酮和吡唑啉酮开始的两步合成3-吡唑基异黄酮的第一个实例。[1,5]-质子转移被用作关键策略。所有3-吡唑基异黄酮3通过传统方法难以合成的,可顺利获得,总收率高达86%。有趣的是,该产物通过色酮核心上的羰基和吡唑啉酮核心上的羟基之间的分子内氢键形成稳定的7元环,从而防止了进一步的酮-烯醇互变异构化。尤其是,该产品的特色在于将两个重要的图案有趣地结合在一起,包括异黄酮和吡唑啉酮亚结构,这在医学化学中可能是有价值的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号