当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fe-catalyzed three-component dicarbofunctionalization of unactivated alkenes with alkyl halides and Grignard reagents
Chemical Science ( IF 7.6 ) Pub Date : 2020-07-24 , DOI: 10.1039/d0sc02127j Lei Liu 1 , Wes Lee 1 , Cassandra R Youshaw 1 , Mingbin Yuan 1 , Michael B Geherty 1 , Peter Y Zavalij 1 , Osvaldo Gutierrez 1
Chemical Science ( IF 7.6 ) Pub Date : 2020-07-24 , DOI: 10.1039/d0sc02127j Lei Liu 1 , Wes Lee 1 , Cassandra R Youshaw 1 , Mingbin Yuan 1 , Michael B Geherty 1 , Peter Y Zavalij 1 , Osvaldo Gutierrez 1
Affiliation
|
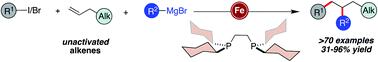
A highly chemoselective iron-catalyzed three-component dicarbofunctionalization of unactivated olefins with alkyl halides (iodides and bromides) and sp2-hybridized Grignard reagents is reported. The reaction operates under fast turnover frequency and tolerates a diverse range of sp2-hybridized nucleophiles (electron-rich and electron-deficient (hetero)aryl and alkenyl Grignard reagents), alkyl halides (tertiary alkyl iodides/bromides and perfluorinated bromides), and unactivated olefins bearing diverse functional groups including tethered alkenes, ethers, protected alcohols, aldehydes, and amines to yield the desired 1,2-alkylarylated products with high regiocontrol. Further, we demonstrate that this protocol is amenable for the synthesis of new (hetero)carbocycles including tetrahydrofurans and pyrrolidines via a three-component radical cascade cyclization/arylation that forges three new C–C bonds.
中文翻译:

Fe 催化卤代烷和格氏试剂对未活化烯烃的三组分二碳官能化
报道了一种高度化学选择性的铁催化的未活化烯烃与烷基卤化物(碘化物和溴化物)和 sp 2杂化格氏试剂的三组分二碳官能化反应。该反应在快速周转频率下进行,可耐受多种 sp 2杂化亲核试剂(富电子和缺电子(杂)芳基和烯基格氏试剂)、烷基卤化物(叔烷基碘化物/溴化物和全氟化溴化物),以及带有多种官能团的未活化烯烃,包括束缚烯烃、醚、受保护的醇、醛和胺,可产生具有高区域控制性的所需 1,2-烷基芳基化产物。此外,我们证明该方案适合通过三组分自由基级联环化/芳基化合成新的(杂)碳环,包括四氢呋喃和吡咯烷,形成三个新的 C-C 键。
更新日期:2020-08-12
中文翻译:

Fe 催化卤代烷和格氏试剂对未活化烯烃的三组分二碳官能化
报道了一种高度化学选择性的铁催化的未活化烯烃与烷基卤化物(碘化物和溴化物)和 sp 2杂化格氏试剂的三组分二碳官能化反应。该反应在快速周转频率下进行,可耐受多种 sp 2杂化亲核试剂(富电子和缺电子(杂)芳基和烯基格氏试剂)、烷基卤化物(叔烷基碘化物/溴化物和全氟化溴化物),以及带有多种官能团的未活化烯烃,包括束缚烯烃、醚、受保护的醇、醛和胺,可产生具有高区域控制性的所需 1,2-烷基芳基化产物。此外,我们证明该方案适合通过三组分自由基级联环化/芳基化合成新的(杂)碳环,包括四氢呋喃和吡咯烷,形成三个新的 C-C 键。











































 京公网安备 11010802027423号
京公网安备 11010802027423号