当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development, Evaluation, and Pharmacokinetic Assessment of Polymeric Microarray Patches for Transdermal Delivery of Vancomycin Hydrochloride.
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-07-24 , DOI: 10.1021/acs.molpharmaceut.0c00431 Delly Ramadon 1, 2 , Andi Dian Permana 1, 3 , Aaron J Courtenay 1, 4 , Maelíosa T C McCrudden 1 , Ismaiel A Tekko 1, 5 , Emma McAlister 1 , Qonita Kurnia Anjani 1 , Emilia Utomo 1 , Helen O McCarthy 1 , Ryan F Donnelly 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-07-24 , DOI: 10.1021/acs.molpharmaceut.0c00431 Delly Ramadon 1, 2 , Andi Dian Permana 1, 3 , Aaron J Courtenay 1, 4 , Maelíosa T C McCrudden 1 , Ismaiel A Tekko 1, 5 , Emma McAlister 1 , Qonita Kurnia Anjani 1 , Emilia Utomo 1 , Helen O McCarthy 1 , Ryan F Donnelly 1
Affiliation
|
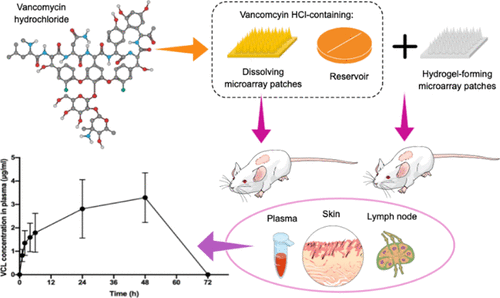
Methicillin-resistant Staphylococcus aureus (MRSA) can cause harmful and potentially deadly infections. Vancomycin remains the first-line antibiotic treatment for MRSA-derived infections. Nevertheless, as a peptide drug, it is poorly absorbed when administered orally because of its high molecular weight and low permeability in the gastrointestinal tract and is therefore administered intravenously for the treatment of systemic diseases. In order to circumvent some of the many drawbacks associated with intravenous injection, other routes of drug delivery should be investigated. One of the strategies which has been employed to enhance transdermal drug delivery is based on microarray patches (MAPs). This work, for the first time, describes successful transdermal delivery of vancomycin hydrochloride (VCL) using dissolving MAPs (DMAPs) and hydrogel-forming MAPs (HFMAPs). VCL was formulated into DMAPs and reservoirs [film dosage forms, lyophilized wafers, and compressed tablets (CSTs)] using excipients such as poly(vinyl pyrrolidone), poly(vinyl alcohol), sodium hyaluronate, d-sorbitol, and glycerol. In this study, HFMAPs were manufactured using aqueous blends containing poly(methylvinyl ether-co-maleic acid) cross-linked by esterification with poly(ethylene glycol). The VCL-loaded CSTs (60% w/w VCL) were the most promising reservoirs to be integrated with HFMAPs based on the physicochemical evaluations performed. Both HFMAPs and DMAPs successfully delivered VCL in ex vivo studies with the percentage of drug that permeated across the neonatal porcine skin recorded at 46.39 ± 8.04 and 7.99 ± 0.98%, respectively. In in vivo studies, the area under the plasma concentration time curve from time zero to infinity (AUC0–∞) values of 162.04 ± 61.84 and 61.01 ± 28.50 μg h/mL were achieved following the application of HFMAPs and DMAPs, respectively. In comparison, the AUC0–∞ of HFMAPs was significantly greater than that of the oral administration control group, which showed an AUC0–∞ of 30.50 ± 9.18 μg h/mL (p < 0.05). This work demonstrates that transdermal delivery of VCL is feasible using DMAPs and HFMAPs and could prove effective in the treatment of infectious diseases caused by MRSA, such as skin and soft tissue infections, lymphatic-related infections, and neonatal sepsis.
中文翻译:

盐酸万古霉素透皮递送聚合物微阵列贴剂的开发,评估和药代动力学评估。
耐甲氧西林金黄色葡萄球菌(MRSA)可能导致有害和潜在的致命感染。万古霉素仍然是针对MRSA衍生感染的一线抗生素治疗。然而,作为肽类药物,由于其高分子量和在胃肠道中的低渗透性,因此口服给药时吸收差,因此静脉内给药用于治疗全身性疾病。为了避免与静脉注射有关的许多弊端中的一些,应该研究其他的药物输送途径。已经用于增强透皮药物递送的策略之一是基于微阵列贴片(MAP)。这项工作首次描述了使用溶解性MAPs(DMAPs)和水凝胶形成MAPs(HFMAPs)成功地透皮递送盐酸万古霉素(VCL)。d-山梨糖醇和甘油。在这项研究中,HFMAPs使用含有聚含水共混物制造的(甲基乙烯基醚-共-马来酸)交联通过酯化与聚(乙二醇)。根据进行的理化评估,VCL负载的CST(60%w / w VCL)是最有希望与HFMAP整合的储层。HFMAPs和DMAPs在离体研究中均成功交付了VCL,跨新生猪皮肤渗透的药物百分比分别为46.39±8.04和7.99±0.98%。在体内研究中,血浆浓度时间曲线下的面积从时间零到无穷大(AUC 0–∞)分别在HFMAPs和DMAPs应用后获得了162.04±61.84和61.01±28.50μgh / mL的值。相比之下,HFMAPs的AUC 0–∞显着大于口服对照组,后者的AUC 0–∞为30.50±9.18μgh / mL(p <0.05)。这项工作表明,使用DMAP和HFMAP进行VCL的透皮给药是可行的,并且可以证明对治疗由MRSA引起的传染病有效,如皮肤和软组织感染,淋巴相关感染和新生儿败血症。
更新日期:2020-09-09
中文翻译:

盐酸万古霉素透皮递送聚合物微阵列贴剂的开发,评估和药代动力学评估。
耐甲氧西林金黄色葡萄球菌(MRSA)可能导致有害和潜在的致命感染。万古霉素仍然是针对MRSA衍生感染的一线抗生素治疗。然而,作为肽类药物,由于其高分子量和在胃肠道中的低渗透性,因此口服给药时吸收差,因此静脉内给药用于治疗全身性疾病。为了避免与静脉注射有关的许多弊端中的一些,应该研究其他的药物输送途径。已经用于增强透皮药物递送的策略之一是基于微阵列贴片(MAP)。这项工作首次描述了使用溶解性MAPs(DMAPs)和水凝胶形成MAPs(HFMAPs)成功地透皮递送盐酸万古霉素(VCL)。d-山梨糖醇和甘油。在这项研究中,HFMAPs使用含有聚含水共混物制造的(甲基乙烯基醚-共-马来酸)交联通过酯化与聚(乙二醇)。根据进行的理化评估,VCL负载的CST(60%w / w VCL)是最有希望与HFMAP整合的储层。HFMAPs和DMAPs在离体研究中均成功交付了VCL,跨新生猪皮肤渗透的药物百分比分别为46.39±8.04和7.99±0.98%。在体内研究中,血浆浓度时间曲线下的面积从时间零到无穷大(AUC 0–∞)分别在HFMAPs和DMAPs应用后获得了162.04±61.84和61.01±28.50μgh / mL的值。相比之下,HFMAPs的AUC 0–∞显着大于口服对照组,后者的AUC 0–∞为30.50±9.18μgh / mL(p <0.05)。这项工作表明,使用DMAP和HFMAP进行VCL的透皮给药是可行的,并且可以证明对治疗由MRSA引起的传染病有效,如皮肤和软组织感染,淋巴相关感染和新生儿败血症。











































 京公网安备 11010802027423号
京公网安备 11010802027423号