当前位置:
X-MOL 学术
›
Z. Anorg. Allg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Two Light‐Metal Dihydrogenisocyanurate Hydrates Linked by Diagonal Relationship: Syntheses, Crystal Structures, and Vibrational Spectra of Li[H2N3C3O3]·1.75 H2O and Mg[H2N3C3O3]2·8 H2O
Zeitschrift für anorganische und allgemeine Chemie ( IF 1.1 ) Pub Date : 2020-07-10 , DOI: 10.1002/zaac.202000015 Olaf Reckeweg 1 , Falk Lissner 1 , Björn Blaschkowski 1 , Peter Gross 2 , Henning A. Höppe 2 , Thomas Schleid 1
Zeitschrift für anorganische und allgemeine Chemie ( IF 1.1 ) Pub Date : 2020-07-10 , DOI: 10.1002/zaac.202000015 Olaf Reckeweg 1 , Falk Lissner 1 , Björn Blaschkowski 1 , Peter Gross 2 , Henning A. Höppe 2 , Thomas Schleid 1
Affiliation
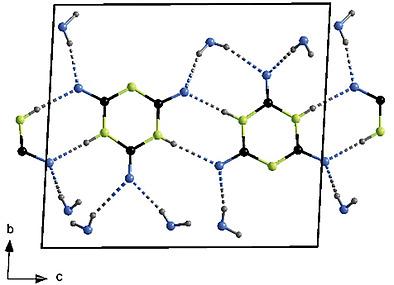
|
Single‐crystalline materials of Li[H2N3C3O3]·1.75 H2O and Mg[H2N3C3O3]2·8 H2O were obtained by dissolving stoichiometric amounts of the respective carbonates with cyanuric acid in boiling water followed by gentle evaporation of excess water after cooling to room temperature. Even though both of these compounds crystallize in the triclinic space group P1 according to X‐ray structure analyses of their colorless and transparent single crystals, they adopt two new different structure types. Li[H2N3C3O3]·1.75 H2O exhibits the unit‐cell parameters a = 884.71(6) pm, b = 905.12(7) pm, c = 964.38(7) pm, α = 67.847(2)°, β = 62.904(2)° and γ = 68.565(2)° (Z = 4), whereas the lattice parameters for Mg[H2N3C3O3]2·8 H2O are a = 691.95(5) pm, b = 1055.06(8) pm, c = 1183.87(9) pm, α = 85.652(2)°, β = 83.439(2)° and γ = 79.814(2)° (Z = 2). In both cases, the singly deprotonated isocyanuric acid forms monovalent anions consisting of cyclic [H2N3C3O3]– units, which are arranged in ribbons typical for most hitherto known monobasic isocyanurate hydrates. The structures are governed by the oxophilic strength of the respective cation which means that they fulfil their oxophilic coordination requirements either solely with water molecules ([Mg(OH2)6]2+ for Mg2+) or with crystal water and one or two direct coordinative contacts to carbonyl oxygen atoms (O(cy)) of [H2N3C3O3]– anions ([(Li(OH2)2–3(O(cy)1–2]+ for Li+). In both structures occur dominant hydrogen bonds N–H···O within the anionic [H2N3C3O3]– ribbons as well as hydrogen bonds O–H···O between these ribbons and the hydrated Li+ and Mg2+ cations.
中文翻译:

对角关系链接的两种轻金属二氢异氰脲酸酯水合物:Li [H2N3C3O3]·1.75 H2O和Mg [H2N3C3O3] 2·8 H2O的合成,晶体结构和振动光谱
Li [H 2 N 3 C 3 O 3 ] · 1.75 H 2 O和Mg [H 2 N 3 C 3 O 3 ] 2 · 8 H 2 O的单晶材料是通过将化学计量的相应碳酸盐溶解于沸水中的氰尿酸,冷却至室温后,将多余的水温和蒸发。即使这两种化合物都在三斜空间群P 1中结晶根据对其无色透明晶体的X射线结构分析,他们采用了两种新的不同结构类型。Li [H 2 N 3 C 3 O 3 ] · 1.75 H 2 O的晶胞参数为a = 884.71(6)pm,b = 905.12(7)pm,c = 964.38(7)pm,α = 67.847( 2)°,β = 62.904(2)°和γ = 68.565(2)°(Z = 4),而Mg [H 2 N 3 C 3 O 3 ] 2 · 8 H的晶格参数2 O为a = 691.95(5)pm,b = 1055.06(8)pm,c = 1183.87(9)pm,α = 85.652(2)°,β = 83.439(2)°和γ = 79.814(2)° (Z = 2)。在这两种情况下,单去质子化的异氰尿酸均形成由环状[H 2 N 3 C 3 O 3 ] –单元排列成条带,对于大多数迄今已知的一元异氰脲酸酯水合物而言,它们通常呈带状排列。结构通过这意味着它们履行亲氧性协调的要求单独要么与水分子各自的阳离子的亲氧性强度支配([镁(OH 2)6 ] 2+为镁2+)或带结晶水和一个或两个引导至羰基的氧原子配位的联系人(O (CY))[H的2 ñ 3 ç 3 ö 3 ] -阴离子([(李(OH 2)2-3 O(CY 2)1-2(〕+对Li +)。在这两种结构中,阴离子[H 2 N 3 C 3 O 3 ] -带内均存在占优势的氢键N–H ·· O ,以及这些带与水合Li +和O之间的氢键OH–·· O Mg 2+阳离子。
更新日期:2020-07-10
中文翻译:

对角关系链接的两种轻金属二氢异氰脲酸酯水合物:Li [H2N3C3O3]·1.75 H2O和Mg [H2N3C3O3] 2·8 H2O的合成,晶体结构和振动光谱
Li [H 2 N 3 C 3 O 3 ] · 1.75 H 2 O和Mg [H 2 N 3 C 3 O 3 ] 2 · 8 H 2 O的单晶材料是通过将化学计量的相应碳酸盐溶解于沸水中的氰尿酸,冷却至室温后,将多余的水温和蒸发。即使这两种化合物都在三斜空间群P 1中结晶根据对其无色透明晶体的X射线结构分析,他们采用了两种新的不同结构类型。Li [H 2 N 3 C 3 O 3 ] · 1.75 H 2 O的晶胞参数为a = 884.71(6)pm,b = 905.12(7)pm,c = 964.38(7)pm,α = 67.847( 2)°,β = 62.904(2)°和γ = 68.565(2)°(Z = 4),而Mg [H 2 N 3 C 3 O 3 ] 2 · 8 H的晶格参数2 O为a = 691.95(5)pm,b = 1055.06(8)pm,c = 1183.87(9)pm,α = 85.652(2)°,β = 83.439(2)°和γ = 79.814(2)° (Z = 2)。在这两种情况下,单去质子化的异氰尿酸均形成由环状[H 2 N 3 C 3 O 3 ] –单元排列成条带,对于大多数迄今已知的一元异氰脲酸酯水合物而言,它们通常呈带状排列。结构通过这意味着它们履行亲氧性协调的要求单独要么与水分子各自的阳离子的亲氧性强度支配([镁(OH 2)6 ] 2+为镁2+)或带结晶水和一个或两个引导至羰基的氧原子配位的联系人(O (CY))[H的2 ñ 3 ç 3 ö 3 ] -阴离子([(李(OH 2)2-3 O(CY 2)1-2(〕+对Li +)。在这两种结构中,阴离子[H 2 N 3 C 3 O 3 ] -带内均存在占优势的氢键N–H ·· O ,以及这些带与水合Li +和O之间的氢键OH–·· O Mg 2+阳离子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号