当前位置:
X-MOL 学术
›
Z. Anorg. Allg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cycloadditions of 1H‐1,3‐Benzazaphospholes with o‐Chloranil
Zeitschrift für anorganische und allgemeine Chemie ( IF 1.4 ) Pub Date : 2020-07-07 , DOI: 10.1002/zaac.202000067 Joachim W. Heinicke 1 , Nidhi Gupta 1, 2 , Peter Mayer 3 , Konstantin Karaghiosoff 3
Zeitschrift für anorganische und allgemeine Chemie ( IF 1.4 ) Pub Date : 2020-07-07 , DOI: 10.1002/zaac.202000067 Joachim W. Heinicke 1 , Nidhi Gupta 1, 2 , Peter Mayer 3 , Konstantin Karaghiosoff 3
Affiliation
|
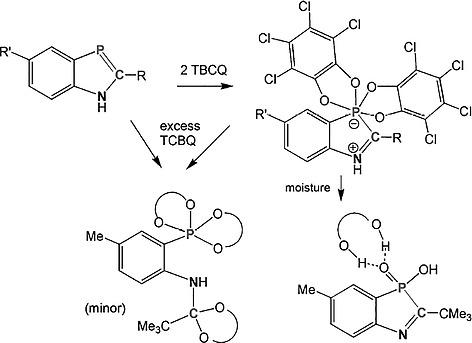
NH‐Functional 1H‐1,3‐benzazaphospholes 1a–1c and o‐chloranil (tetrachloro‐o‐benzoquinone ‐ TCBQ) undergo rapid [1+4]‐cycloaddition in a 1:2 molar ratio to give 2a–2c as high‐melting zwitterionic σ6λ5‐phosphorus compounds. In the case of 2a the yield is high (rel. to TCBQ) even if the reactants were used in a 1:0.5 molar ratio. For the 2‐tert‐butyl‐substituted compounds 2b and 2c the yields were significantly lower, in part by unidentified byproducts. Addition of excess TCBQ to crude 2c containing unconverted 1c did not increase but strongly decrease the amount of 2c. Crystallization and XRD analysis led to detection of a minor side or consecutive product 3c, formally corresponding to P=C bond cleavage and [1+4] cycloaddition of three equivalents TCBQ, two at the phosphinidene and one at the carbene end. NMR spectroscopic data of 2a–2c including conclusive 13C data for 2a give evidence of the structures of the new compounds.
中文翻译:

1H-1,3-苯并氮杂唑与邻氯腈的环加成
NH功能性1H -1,3-苯并氮杂唑1a-1c和邻氯苯甲腈(四氯邻苯醌TCBQ)以1:2摩尔比快速[1 + 4]环加成,得到2a - 2c为高熔化两性离子σ 6 λ 5 -磷化合物。在2a的情况下,即使以1:0.5的摩尔比使用反应物,收率也很高(相对于TCBQ)。对于2-叔丁基取代的化合物2b和2c,产率显着降低,部分原因是未鉴定的副产物。在原油2c中添加过量的TCBQ包含未转化的1c的含量没有增加,但大大降低了2c的含量。结晶和XRD分析导致检测到次要的副产物或连续产物3c,形式上对应于P = C键断裂和三个等价TCBQ的[1 + 4]环加成,两个在次膦基上,一个在卡宾末端。的NMR光谱数据2a中-图2c包括确凿13为C数据2a中的新化合物的结构的提供证据。
更新日期:2020-07-07
中文翻译:

1H-1,3-苯并氮杂唑与邻氯腈的环加成
NH功能性1H -1,3-苯并氮杂唑1a-1c和邻氯苯甲腈(四氯邻苯醌TCBQ)以1:2摩尔比快速[1 + 4]环加成,得到2a - 2c为高熔化两性离子σ 6 λ 5 -磷化合物。在2a的情况下,即使以1:0.5的摩尔比使用反应物,收率也很高(相对于TCBQ)。对于2-叔丁基取代的化合物2b和2c,产率显着降低,部分原因是未鉴定的副产物。在原油2c中添加过量的TCBQ包含未转化的1c的含量没有增加,但大大降低了2c的含量。结晶和XRD分析导致检测到次要的副产物或连续产物3c,形式上对应于P = C键断裂和三个等价TCBQ的[1 + 4]环加成,两个在次膦基上,一个在卡宾末端。的NMR光谱数据2a中-图2c包括确凿13为C数据2a中的新化合物的结构的提供证据。



























 京公网安备 11010802027423号
京公网安备 11010802027423号