当前位置:
X-MOL 学术
›
Pept. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Using 19F NMR and two‐level factorial design to explore thiol‐fluoride substitution in hexafluorobenzene and its application in peptide stapling and cyclisation
Peptide Science ( IF 1.5 ) Pub Date : 2020-07-09 , DOI: 10.1002/pep2.24182 Paolo Dognini 1 , Patrick M. Killoran 2 , George S. Hanson 1, 3 , Lewis Halsall 1 , Talhat Chaudhry 1 , Zasharatul Islam 1 , Francesca Giuntini 1 , Christopher R. Coxon 3
Peptide Science ( IF 1.5 ) Pub Date : 2020-07-09 , DOI: 10.1002/pep2.24182 Paolo Dognini 1 , Patrick M. Killoran 2 , George S. Hanson 1, 3 , Lewis Halsall 1 , Talhat Chaudhry 1 , Zasharatul Islam 1 , Francesca Giuntini 1 , Christopher R. Coxon 3
Affiliation
|
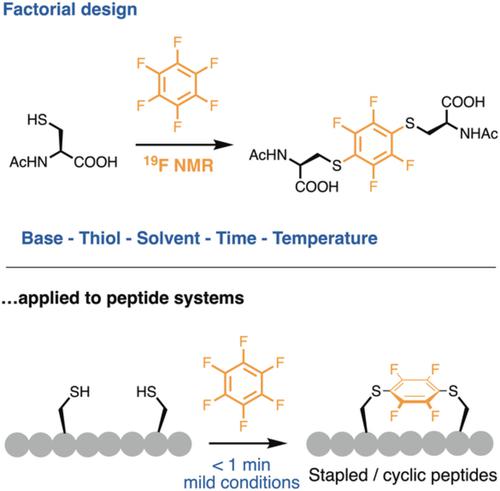
Hexafluorobenzene undergoes 1,4‐selective thiol‐fluoride disubstitution and is an attractive disulfide crosslinking reagent for peptide cyclisation and stapling. Little attention has been directed toward understanding the scope of this reaction. Traditional reaction optimisation relies on a one‐variable‐at‐a‐time approach, which can exclude important combined effects of reaction variables. This study initially explored base and solvent effects to inform a subsequent two‐level factorial design approach to understand how to control the reactivity and product selectivity in a model reaction of hexafluorobenzene. We describe new conditions that selectively afford higher order substitution products for example, 1,2,4,5‐tetrasubstitution, making hexafluorobenzene a possible suitable scaffold for future branched or multicyclic peptide systems. Moreover, our new conditions provide an improved rapid (<1 minute) and selective peptide disulfide stapling and cyclisation approach under peptide‐compatible conditions.
中文翻译:

使用19F NMR和两级因子设计研究六氟苯中的巯基氟化物取代及其在肽装订和环化中的应用
六氟苯经历1,4-选择性硫醇-氟化物分解,是一种有吸引力的二硫键交联剂,用于肽的环化和装订。很少有人注意了解这一反应的范围。传统的反应优化依赖于一次可变的方法,该方法可以排除反应变量的重要综合影响。这项研究最初探索了碱和溶剂的影响,以为随后的两级因子设计方法提供信息,以了解如何在六氟苯模型反应中控制反应性和产物选择性。我们描述了选择性提供更高阶取代产物(例如1,2,4,5-四取代)的新条件,从而使六氟苯可能成为未来分支或多环肽系统的合适支架。此外,
更新日期:2020-07-09
中文翻译:

使用19F NMR和两级因子设计研究六氟苯中的巯基氟化物取代及其在肽装订和环化中的应用
六氟苯经历1,4-选择性硫醇-氟化物分解,是一种有吸引力的二硫键交联剂,用于肽的环化和装订。很少有人注意了解这一反应的范围。传统的反应优化依赖于一次可变的方法,该方法可以排除反应变量的重要综合影响。这项研究最初探索了碱和溶剂的影响,以为随后的两级因子设计方法提供信息,以了解如何在六氟苯模型反应中控制反应性和产物选择性。我们描述了选择性提供更高阶取代产物(例如1,2,4,5-四取代)的新条件,从而使六氟苯可能成为未来分支或多环肽系统的合适支架。此外,









































 京公网安备 11010802027423号
京公网安备 11010802027423号