当前位置:
X-MOL 学术
›
Pept. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Proteomimetic zinc finger domains with modified metal‐binding β‐turns
Peptide Science ( IF 1.5 ) Pub Date : 2020-06-07 , DOI: 10.1002/pep2.24177 Shilpa R Rao 1 , W Seth Horne 1
Peptide Science ( IF 1.5 ) Pub Date : 2020-06-07 , DOI: 10.1002/pep2.24177 Shilpa R Rao 1 , W Seth Horne 1
Affiliation
|
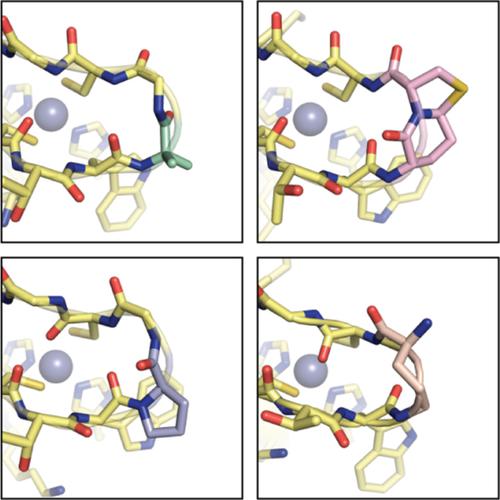
The mimicry of protein tertiary folds by chains artificial in backbone chemical composition leads to proteomimetic analogues with potential utility as bioactive agents and as tools, to shed light on biomacromolecule behavior. Notable successes toward such molecules have been achieved; however, as protein structural diversity is vast, design principles must be continually honed as they are applied to new prototype folding patterns. One specific structure where a gap remains in understanding how to effectively generate modified backbone analogues is the metal‐binding β‐turn found in zinc finger domains. The literature precedent suggests several factors that may act in concert, including the artificial moiety used to modify the turn, the sequence in which it is applied, and modifications present elsewhere in the domain. Here, we report efforts to gain insights into these issues and leverage these insights to construct a zinc finger mimetic with backbone modifications throughout its constituent secondary structures. We first conduct a systematic comparison of four turn mimetics in a common host sequence, quantifying relative efficacy for use in a metal‐binding context. We go on to construct a proteomimetic zinc finger domain in which the helix, strands, and turn are simultaneously modified, resulting in a variant with 23% artificial residues, a tertiary fold indistinguishable from the prototype, and a folded stability comparable to the natural backbone on which the variant is based. Collectively, the results reported provide new insights into the effects of backbone modification on the structure and stability of metal‐binding domains and help inform the design of metalloprotein mimetics.
中文翻译:

具有修饰的金属结合β-转角的蛋白质模拟锌指结构域
主链化学成分中人工链对蛋白质三级折叠的模拟导致蛋白质模拟类似物具有作为生物活性剂和工具的潜在效用,以阐明生物大分子的行为。这些分子已经取得了显着的成功。然而,由于蛋白质结构的多样性是巨大的,设计原则在应用于新的原型折叠模式时必须不断磨练。在理解如何有效地生成修饰的骨架类似物方面仍然存在差距的一种特定结构是在锌指结构域中发现的金属结合 β-转角。文献先例提出了几个可能协同作用的因素,包括用于修改转角的人工部分、应用的顺序以及域中其他地方的修改。这里,我们报告了为深入了解这些问题所做的努力,并利用这些见解来构建锌指模拟物,其整个二级结构都具有骨架修饰。我们首先对常见宿主序列中的四个转角模拟物进行系统比较,量化在金属结合环境中使用的相对功效。我们继续构建了一个蛋白质模拟锌指结构域,其中螺旋、链和转角同时被修饰,产生具有 23% 人工残基的变体、与原型无法区分的三级折叠以及与天然骨架相当的折叠稳定性变体所基于的。总的来说,
更新日期:2020-06-07
中文翻译:

具有修饰的金属结合β-转角的蛋白质模拟锌指结构域
主链化学成分中人工链对蛋白质三级折叠的模拟导致蛋白质模拟类似物具有作为生物活性剂和工具的潜在效用,以阐明生物大分子的行为。这些分子已经取得了显着的成功。然而,由于蛋白质结构的多样性是巨大的,设计原则在应用于新的原型折叠模式时必须不断磨练。在理解如何有效地生成修饰的骨架类似物方面仍然存在差距的一种特定结构是在锌指结构域中发现的金属结合 β-转角。文献先例提出了几个可能协同作用的因素,包括用于修改转角的人工部分、应用的顺序以及域中其他地方的修改。这里,我们报告了为深入了解这些问题所做的努力,并利用这些见解来构建锌指模拟物,其整个二级结构都具有骨架修饰。我们首先对常见宿主序列中的四个转角模拟物进行系统比较,量化在金属结合环境中使用的相对功效。我们继续构建了一个蛋白质模拟锌指结构域,其中螺旋、链和转角同时被修饰,产生具有 23% 人工残基的变体、与原型无法区分的三级折叠以及与天然骨架相当的折叠稳定性变体所基于的。总的来说,











































 京公网安备 11010802027423号
京公网安备 11010802027423号