当前位置:
X-MOL 学术
›
Pept. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of residue insertion on the stability of polyproline‐I and II structures: Circular dichroism spectroscopic analyses of block‐type oligo‐prolines (Pro)m‐Gly/Ala‐(Pro)n
Peptide Science ( IF 1.5 ) Pub Date : 2020-04-27 , DOI: 10.1002/pep2.24170 Sachiro Kakinoki 1, 2 , Makoto Kitamura 3 , Yuri Noguchi 4 , Yuki Arichi 4
Peptide Science ( IF 1.5 ) Pub Date : 2020-04-27 , DOI: 10.1002/pep2.24170 Sachiro Kakinoki 1, 2 , Makoto Kitamura 3 , Yuri Noguchi 4 , Yuki Arichi 4
Affiliation
|
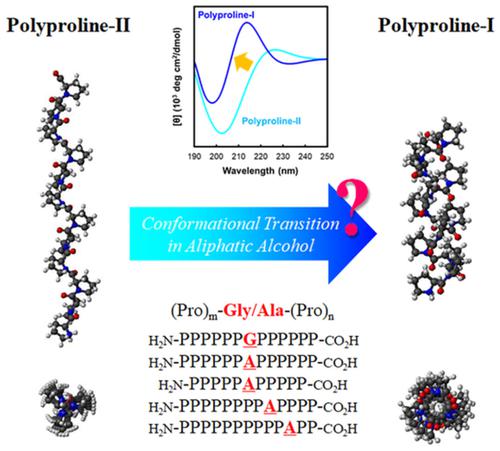
The molecular conformation of oligo‐proline peptides composed of two oligo‐proline block sequences and a non‐proline linker residue, designated as (Pro)
m‐Gly/Ala‐(Pro)
n peptides, was analyzed by circular dichroism (CD) spectroscopy. The CD spectra in water and trifluoroethanol indicated that the two oligo‐proline blocks were separated by an inserted residue independent of polyproline‐II (PP‐II). In addition, the stability of the (Pro)
m‐Gly/Ala‐(Pro)
n peptides was analyzed using a conformational transition system, during transition from PP‐II to polyproline‐I (PP‐I) in aliphatic alcohols, methanol (MeOH), and 1‐propanol (1‐PrOH). Interestingly, the PP‐II/PP‐I transition was inhibited after a Gly/Ala was inserted at the center of the oligo‐proline; the inhibitory effect of Ala was stronger than that of Gly. When the position of the inserted Ala moved towards the C‐terminal, the (Pro)
m‐Gly/Ala‐(Pro)
n peptides displayed a PP‐II/PP‐I transition in 1‐PrOH. Our results confirmed that (Pro)
m‐Gly/Ala‐(Pro)
n peptides prefer to form PP‐II hairpin conformations even in MeOH and 1‐PrOH. Thus, our findings suggest that the insertion of Gly/Ala acts as a stabilizer in PP‐II in proline‐rich peptides.
中文翻译:

残基插入对Polyproline-I和II结构稳定性的影响:嵌段二聚脯氨酸(Pro)m-Gly / Ala-(Pro)n的圆二色光谱分析
通过圆二色性(CD)光谱分析了由两个寡脯氨酸嵌段序列和一个非脯氨酸接头残基组成的寡脯氨酸肽的分子构象,命名为(Pro)m -Gly / Ala-(Pro)n肽。水和三氟乙醇中的CD光谱表明,两个寡脯氨酸嵌段被插入的残基分开,而残基独立于聚脯氨酸II(PP-II)。此外,(Pro)m ‐Gly / Ala‐(Pro)n的稳定性 在脂肪族醇,甲醇(MeOH)和1-丙醇(1-PrOH)中从PP-II到聚脯氨酸-I(PP-I)的过渡过程中,使用构象过渡系统分析了多肽。有趣的是,在寡脯氨酸的中心插入了Gly / Ala后,PP-II / PP-I的转移受到抑制。丙氨酸的抑制作用强于甘氨酸。当插入的Ala的位置向C端移动时,(Pro)m -Gly / Ala-(Pro)n肽在1-PrOH中显示PP-II / PP-I过渡。我们的结果证实(Pro)m ‐Gly / Ala‐(Pro)n 肽甚至在MeOH和1-PrOH中更倾向于形成PP-II发夹构象。因此,我们的发现表明,富含脯氨酸的肽中的Gly / Ala插入可作为PP-II中的稳定剂。
更新日期:2020-04-27
中文翻译:

残基插入对Polyproline-I和II结构稳定性的影响:嵌段二聚脯氨酸(Pro)m-Gly / Ala-(Pro)n的圆二色光谱分析
通过圆二色性(CD)光谱分析了由两个寡脯氨酸嵌段序列和一个非脯氨酸接头残基组成的寡脯氨酸肽的分子构象,命名为(Pro)m -Gly / Ala-(Pro)n肽。水和三氟乙醇中的CD光谱表明,两个寡脯氨酸嵌段被插入的残基分开,而残基独立于聚脯氨酸II(PP-II)。此外,(Pro)m ‐Gly / Ala‐(Pro)n的稳定性 在脂肪族醇,甲醇(MeOH)和1-丙醇(1-PrOH)中从PP-II到聚脯氨酸-I(PP-I)的过渡过程中,使用构象过渡系统分析了多肽。有趣的是,在寡脯氨酸的中心插入了Gly / Ala后,PP-II / PP-I的转移受到抑制。丙氨酸的抑制作用强于甘氨酸。当插入的Ala的位置向C端移动时,(Pro)m -Gly / Ala-(Pro)n肽在1-PrOH中显示PP-II / PP-I过渡。我们的结果证实(Pro)m ‐Gly / Ala‐(Pro)n 肽甚至在MeOH和1-PrOH中更倾向于形成PP-II发夹构象。因此,我们的发现表明,富含脯氨酸的肽中的Gly / Ala插入可作为PP-II中的稳定剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号