当前位置:
X-MOL 学术
›
Pept. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One‐pot peptide cleavage and macrocyclization through direct amidation using triazabicyclodecene
Peptide Science ( IF 1.5 ) Pub Date : 2020-03-24 , DOI: 10.1002/pep2.24161 Jennifer L. Hickey 1 , Songnian Lin 1
Peptide Science ( IF 1.5 ) Pub Date : 2020-03-24 , DOI: 10.1002/pep2.24161 Jennifer L. Hickey 1 , Songnian Lin 1
Affiliation
|
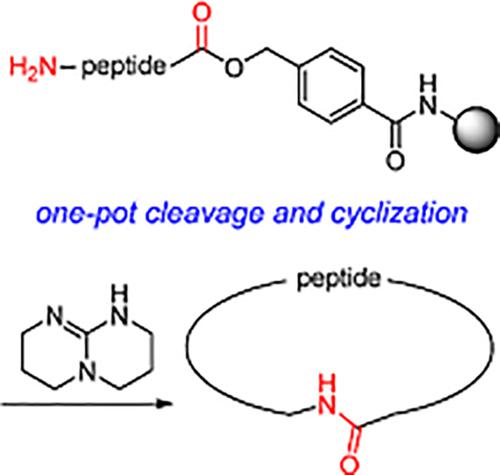
We have developed a novel protocol for a one‐pot cleavage from solid support and subsequent cyclization to form small macrocyclic peptides. Synthesized on a 4‐hydroxymethyl benzamide (HMBA) resin, the peptides are cleaved from the solid support through a 1,5,7‐triazabicyclo[4.4.0]dec‐5‐ene (TBD) assisted acyl transfer reaction. The resulting linear peptides then undergo an intramolecular cyclization, presumably upon in situ attack of the N‐terminal amine on the TBD‐activated ester. This protocol requires catalytic quantities of TBD and eliminates the need for orthogonal side chain deprotection strategies and ether trituration/work‐up steps, streamlining the synthesis of head‐to‐tail macrocyclic peptides.
中文翻译:

使用三氮杂双环癸烯通过直接酰胺化作用进行单锅肽裂解和大环化
我们已经开发了一种从固相支持物进行单锅裂解并随后环化形成小的大环肽的新颖方案。这些肽是在4-羟甲基苯甲酰胺(HMBA)树脂上合成的,通过1,5,7-三氮杂双环[4.4.0] dec-5-烯(TBD)辅助的酰基转移反应从固相支持物上裂解下来。然后,可能是在N末端胺对TBD活化酯的原位攻击后,所产生的线性肽进行了分子内环化。该方案需要催化量的TBD,并且不需要正交的侧链去保护策略和醚研磨/后处理步骤,从而简化了首尾大环肽的合成。
更新日期:2020-03-24
中文翻译:

使用三氮杂双环癸烯通过直接酰胺化作用进行单锅肽裂解和大环化
我们已经开发了一种从固相支持物进行单锅裂解并随后环化形成小的大环肽的新颖方案。这些肽是在4-羟甲基苯甲酰胺(HMBA)树脂上合成的,通过1,5,7-三氮杂双环[4.4.0] dec-5-烯(TBD)辅助的酰基转移反应从固相支持物上裂解下来。然后,可能是在N末端胺对TBD活化酯的原位攻击后,所产生的线性肽进行了分子内环化。该方案需要催化量的TBD,并且不需要正交的侧链去保护策略和醚研磨/后处理步骤,从而简化了首尾大环肽的合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号