当前位置:
X-MOL 学术
›
Pept. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Investigation of the structure‐activity relationship in ponericin L1 from Neoponera goeldii
Peptide Science ( IF 1.5 ) Pub Date : 2020-03-31 , DOI: 10.1002/pep2.24162 Alexandria S Senetra 1 , Matthew R Necelis 1 , Gregory A Caputo 1, 2
Peptide Science ( IF 1.5 ) Pub Date : 2020-03-31 , DOI: 10.1002/pep2.24162 Alexandria S Senetra 1 , Matthew R Necelis 1 , Gregory A Caputo 1, 2
Affiliation
|
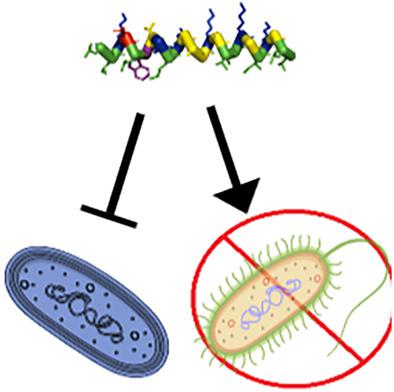
Naturally derived antimicrobial peptides have been an area of great interest because of high selectivity against bacterial targets over host cells and the limited development of bacterial resistance to these molecules throughout evolution. There are also a significant number of venom‐derived peptides that exhibit antimicrobial activity in addition to activity against mammals or other organisms. Many venom peptides share the same net cationic, amphiphilic nature as host‐defense peptides, making them an attractive target for development as potential antibacterial agents. The peptide ponericin L1 derived from Neoponera goeldii was used as a model to investigate the role of cationic residues and net charge on peptide activity. Using a combination of spectroscopic and microbiological approaches, the role of cationic residues and net charge on antibacterial activity, lipid bilayer interactions, and bilayer and membrane permeabilization were investigated. The L1 peptide and derivatives all showed enhanced binding to lipid vesicles containing anionic lipids, but still bound to zwitterionic vesicles. None of the derivatives were especially effective at permeabilizing lipid bilayers in model vesicles, in‐tact Escherichia coli, or human red blood cells. Taken together the results indicate that the lack of facial amphiphilicity regarding charge segregation may impact the ability of the L1 peptides to effectively permeabilize bilayers despite effective binding. Additionally, increasing the net charge of the peptide by replacing the lone anionic residue with either Gln or Lys dramatically improved efficacy against several bacterial strains without increasing hemolytic activity.
中文翻译:

戈氏新珊瑚素L1构效关系研究
天然衍生的抗菌肽一直是人们非常感兴趣的领域,因为它对宿主细胞上的细菌靶标具有高选择性,并且在整个进化过程中细菌对这些分子的耐药性发展有限。还有大量毒液衍生肽除了具有针对哺乳动物或其他生物体的活性外,还具有抗菌活性。许多毒液肽与宿主防御肽具有相同的净阳离子、两亲性质,这使它们成为潜在抗菌剂开发的有吸引力的目标。以来源于戈氏新波纳菌的肽ponricin L1为模型,研究了阳离子残基和净电荷对肽活性的作用。结合光谱学和微生物学方法,研究了阳离子残基和净电荷对抗菌活性、脂质双层相互作用以及双层和膜通透性的作用。 L1肽和衍生物均显示出与含有阴离子脂质的脂质囊泡的结合增强,但仍与两性离子囊泡结合。这些衍生物在透化模型囊泡、完整大肠杆菌或人红细胞中的脂质双层方面都不是特别有效。总而言之,结果表明,尽管有效结合,但电荷分离方面缺乏表面两亲性可能会影响 L1 肽有效渗透双层的能力。此外,通过用 Gln 或 Lys 取代单独的阴离子残基来增加肽的净电荷,可显着提高针对多种细菌菌株的功效,而不会增加溶血活性。
更新日期:2020-03-31
中文翻译:

戈氏新珊瑚素L1构效关系研究
天然衍生的抗菌肽一直是人们非常感兴趣的领域,因为它对宿主细胞上的细菌靶标具有高选择性,并且在整个进化过程中细菌对这些分子的耐药性发展有限。还有大量毒液衍生肽除了具有针对哺乳动物或其他生物体的活性外,还具有抗菌活性。许多毒液肽与宿主防御肽具有相同的净阳离子、两亲性质,这使它们成为潜在抗菌剂开发的有吸引力的目标。以来源于戈氏新波纳菌的肽ponricin L1为模型,研究了阳离子残基和净电荷对肽活性的作用。结合光谱学和微生物学方法,研究了阳离子残基和净电荷对抗菌活性、脂质双层相互作用以及双层和膜通透性的作用。 L1肽和衍生物均显示出与含有阴离子脂质的脂质囊泡的结合增强,但仍与两性离子囊泡结合。这些衍生物在透化模型囊泡、完整大肠杆菌或人红细胞中的脂质双层方面都不是特别有效。总而言之,结果表明,尽管有效结合,但电荷分离方面缺乏表面两亲性可能会影响 L1 肽有效渗透双层的能力。此外,通过用 Gln 或 Lys 取代单独的阴离子残基来增加肽的净电荷,可显着提高针对多种细菌菌株的功效,而不会增加溶血活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号