当前位置:
X-MOL 学术
›
Bull. Korean Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Facile Synthesis of Functionalized 1,4‐Benzodiazepine‐3‐One‐5‐Acetates via [4 + 3]‐Annulation of Azaoxyallyl Cations With 2‐Aminophenyl α,β‐Unsaturated Esters
Bulletin of the Korean Chemical Society ( IF 1.7 ) Pub Date : 2020-07-08 , DOI: 10.1002/bkcs.12060 Hyun Sun Jang 1 , Yong Il Kwon 1 , Sung‐Gon Kim 1
Bulletin of the Korean Chemical Society ( IF 1.7 ) Pub Date : 2020-07-08 , DOI: 10.1002/bkcs.12060 Hyun Sun Jang 1 , Yong Il Kwon 1 , Sung‐Gon Kim 1
Affiliation
|
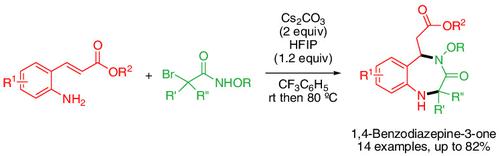
A metal‐free [4 + 3]‐annulation of α‐halohydroxamates with 2‐aminophenyl α,β‐unsaturated esters has been developed for the construction of seven‐membered 1,4‐benzodiazepine‐3‐one‐5‐acetates in moderate to good yields (up to 82% yield). The annulation involved the cascade reaction of an generation of azaoxyallyl cation, aza‐addition to this azaoxyallyl cation, and intramolecular aza‐Michael reaction to yield 1,4‐benzodiazepine‐3‐one‐5‐acetates.
中文翻译:

通过[4 + 3]与2-氨基苯基α,β-不饱和酯的氮杂烯丙基阳离子的环化反应,可轻松合成功能化的1,4-苯并二氮杂-3-3-5
已开发了一种无金属的α-卤代异羟肟酸酯与2个氨基苯基α,β-不饱和酯的[4 + 3]环化反应,可适度地构建7元1,4-苯并二氮杂-3--3-5乙酸酯达到良好的产量(高达82%的产量)。环状反应涉及生成氮杂氧基烯丙基阳离子的级联反应,向该氮杂氧基烯丙基阳离子的氮杂加成反应以及分子内的氮杂迈克尔反应,生成1,4-苯并二氮杂-3-1-5乙酸酯。
更新日期:2020-07-08
中文翻译:

通过[4 + 3]与2-氨基苯基α,β-不饱和酯的氮杂烯丙基阳离子的环化反应,可轻松合成功能化的1,4-苯并二氮杂-3-3-5
已开发了一种无金属的α-卤代异羟肟酸酯与2个氨基苯基α,β-不饱和酯的[4 + 3]环化反应,可适度地构建7元1,4-苯并二氮杂-3--3-5乙酸酯达到良好的产量(高达82%的产量)。环状反应涉及生成氮杂氧基烯丙基阳离子的级联反应,向该氮杂氧基烯丙基阳离子的氮杂加成反应以及分子内的氮杂迈克尔反应,生成1,4-苯并二氮杂-3-1-5乙酸酯。



























 京公网安备 11010802027423号
京公网安备 11010802027423号