当前位置:
X-MOL 学术
›
Bull. Korean Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Application of New Calix[4]Arenes‐Containing (R)‐Phenylglycinol Chiral Stationary Phases for Enantioseparation
Bulletin of the Korean Chemical Society ( IF 2.3 ) Pub Date : 2020-07-06 , DOI: 10.1002/bkcs.12063 Kyu Sung Heo 1 , Jae Jeong Ryoo 2
Bulletin of the Korean Chemical Society ( IF 2.3 ) Pub Date : 2020-07-06 , DOI: 10.1002/bkcs.12063 Kyu Sung Heo 1 , Jae Jeong Ryoo 2
Affiliation
|
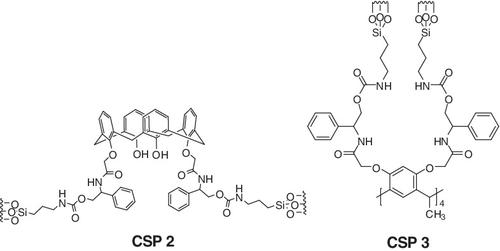
N‐3,5‐Dinitrobenzoyl‐(R)‐phenylglycinol silylation product was used as a high‐performance liquid chromatography chiral stationary phase (CSP 1) for the resolution of various racemic samples, and some racemic samples were successfully separated. In this study, instead of the commonly used π‐acidic acyl chloride, calix[4]arenes were introduced to prepare two phenylglycinol CSPs (CSP 2, CSP 3). CSP 3 showed similar separation patterns as CSP 1 but different characteristics in specific samples. The newly prepared CSP 3 separated 10 of 13 π‐acidic, π‐basic, and oxazolidinone chiral samples and was especially useful for separating chiral oxazolidinones. In comparison between CSP 2 and CSP 3, CSP 2 separated fewer chiral samples than CSP 3 because of poor cavity and steric interactions. The newly developed C‐methylcalix[4]resorcinarene derived CSP (CSP 3) will be a good model for the development of new stationary phase of this calixarene series.
中文翻译:

对映异构体分离的新杯含[4]戊烯的(R)-苯基甘醇手性固定相的合成与应用
N -3,5-二硝基苯甲酰基-(R)-苯基甘氨醇甲硅烷基化产物用作高效液相色谱手性固定相(CSP 1),用于分离各种外消旋样品,并成功分离了一些外消旋样品。在这项研究中,代替常用的π-酸性酰氯,引入杯[4]芳烃来制备两种苯基甘醇CSP(CSP 2,CSP 3)。CSP 3的分离模式与CSP 1相似,但特定样品的特征不同。新制备的CSP 3分离了13个π-酸性,π-碱性和恶唑烷酮手性样品中的10个,特别适用于分离手性恶唑烷酮。在CSP 2和CSP 3之间进行比较,由于差的空腔和空间相互作用,CSP 2分离的手性样品少于CSP 3。新开发的C‐methylcalix [4]间苯二甲烯衍生的CSP(CSP 3)将是开发此杯芳烃系列新固定相的良好模型。
更新日期:2020-07-06
中文翻译:

对映异构体分离的新杯含[4]戊烯的(R)-苯基甘醇手性固定相的合成与应用
N -3,5-二硝基苯甲酰基-(R)-苯基甘氨醇甲硅烷基化产物用作高效液相色谱手性固定相(CSP 1),用于分离各种外消旋样品,并成功分离了一些外消旋样品。在这项研究中,代替常用的π-酸性酰氯,引入杯[4]芳烃来制备两种苯基甘醇CSP(CSP 2,CSP 3)。CSP 3的分离模式与CSP 1相似,但特定样品的特征不同。新制备的CSP 3分离了13个π-酸性,π-碱性和恶唑烷酮手性样品中的10个,特别适用于分离手性恶唑烷酮。在CSP 2和CSP 3之间进行比较,由于差的空腔和空间相互作用,CSP 2分离的手性样品少于CSP 3。新开发的C‐methylcalix [4]间苯二甲烯衍生的CSP(CSP 3)将是开发此杯芳烃系列新固定相的良好模型。











































 京公网安备 11010802027423号
京公网安备 11010802027423号