Journal of Environmental Chemical Engineering ( IF 7.4 ) Pub Date : 2020-05-23 , DOI: 10.1016/j.jece.2020.103901 Şahika Sena Bayazit , Selin Şahin
|
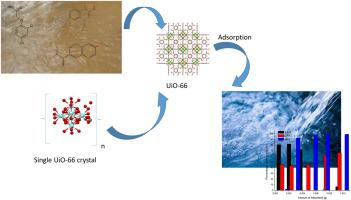
UiO-66 is one of the most applied metal organic frameworks on environmental remediation. In this study, UiO-66 was prepared and modified it by acid treatment. Acid treatment causes defected sites in the crystals. These defected sites provide increasing the adsorption capacities of crystals. The surface properties of prepared UiO-66 crystals were examined by X-ray diffraction spectroscopy (XRD), Fourier transform Infrared spectroscopy (FTIR), BET surface area analysis, particle size distribution analysis, and Scanning electron microscopy (SEM), respectively. 2,4-dichlorophenoxy acetic acid (2,4-D), ciprofloxacin (CPX) and naproxen (NPX) were adsorbed on UiO-66 crystals. Some adsorption variables were examined. These variables are amount of adsorbent, pH of adsorbate solution, initial adsorbate concentration, and contact time. The equilibrium times were determined as 120 min for 2,4-D, 45 min for CPX and 120 min for NPX. Pseudo second order (PSO), fractional power and Elovich kinetic models were calculated. Additionally, Langmuir, Freundlich, Sips and Radke-Prausnitz isotherms were also calculated. PSO kinetic model has been suited the adsorption of all three micropollutants. Langmuir isotherm has been fitted to 2,4-D, CPX and NPX adsorption. The calculated maximum adsorption uptake values of 2,4-D, CPX and NPX are 370.37, 111.71 and 43.86 mg/g, respectively.
中文翻译:

酸调节锆基金属有机骨架,用于去除有机微量污染物
UiO-66是在环境修复方面应用最广泛的金属有机框架之一。在本研究中,制备了UiO-66并通过酸处理对其进行了改性。酸处理会在晶体中造成缺陷部位。这些缺陷部位增加了晶体的吸附能力。分别通过X射线衍射光谱(XRD),傅立叶变换红外光谱(FTIR),BET表面积分析,粒度分布分析和扫描电子显微镜(SEM)检查制备的UiO-66晶体的表面性能。2,4-二氯苯氧基乙酸(2,4-D),环丙沙星(CPX)和萘普生(NPX)吸附在UiO-66晶体上。检查了一些吸附变量。这些变量是吸附剂的量,吸附剂溶液的pH,初始吸附剂浓度和接触时间。平衡时间确定为2,4-D为120分钟,CPX为45分钟,NPX为120分钟。计算了伪二阶(PSO),分数功率和Elovich动力学模型。此外,还计算了Langmuir,Freundlich,Sips和Radke-Prausnitz等温线。PSO动力学模型适用于所有三种微量污染物的吸附。Langmuir等温线已适合2,4-D,CPX和NPX吸附。计算出的2,4-D,CPX和NPX的最大吸附值分别为370.37、111.71和43.86 mg / g。PSO动力学模型适用于所有三种微量污染物的吸附。Langmuir等温线已适合2,4-D,CPX和NPX吸附。计算出的2,4-D,CPX和NPX的最大吸附值分别为370.37、111.71和43.86 mg / g。PSO动力学模型适用于所有三种微量污染物的吸附。Langmuir等温线已适合2,4-D,CPX和NPX吸附。计算出的2,4-D,CPX和NPX的最大吸附值分别为370.37、111.71和43.86 mg / g。









































 京公网安备 11010802027423号
京公网安备 11010802027423号