Cellular and Molecular Gastroenterology and Hepatology ( IF 7.1 ) Pub Date : 2020-07-19 , DOI: 10.1016/j.jcmgh.2020.07.006 Ali Saeed 1 , Paulina Bartuzi 2 , Janette Heegsma 3 , Daphne Dekker 2 , Niels Kloosterhuis 2 , Alain de Bruin 4 , Johan W Jonker 5 , Bart van de Sluis 2 , Klaas Nico Faber 3
|
Background & Aims
Systemic retinol (vitamin A) homeostasis is controlled by the liver, involving close collaboration between hepatocytes and hepatic stellate cells (HSCs). Genetic variants in retinol metabolism (PNPLA3 and HSD17B13) are associated with non-alcoholic fatty liver disease (NAFLD) and disease progression. Still, little mechanistic details are known about hepatic vitamin A metabolism in NAFLD, which may affect carbohydrate and lipid metabolism, inflammation, oxidative stress and the development of fibrosis and cancer, e.g. all risk factors of NAFLD.
Methods
Here, we analyzed vitamin A metabolism in 2 mouse models of NAFLD; mice fed a high-fat, high-cholesterol (HFC) diet and Leptinob mutant (ob/ob) mice.
Results
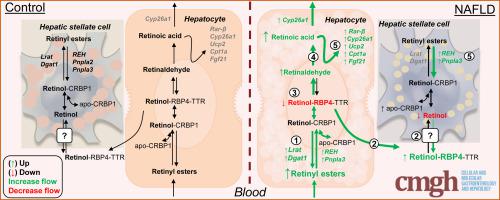
Hepatic retinol and retinol binding protein 4 (RBP4) levels were significantly reduced in both mouse models of NAFLD. In contrast, hepatic retinyl palmitate levels (the vitamin A storage form) were significantly elevated in these mice. Transcriptome analysis revealed a hyperdynamic state of hepatic vitamin A metabolism, with enhanced retinol storage and metabolism (upregulated Lrat, Dgat1, Pnpla3, Raldh’s and RAR/RXR-target genes) in fatty livers, in conjunction with induced hepatic inflammation (upregulated Cd68, Tnfα, Nos2, Il1β, Il-6) and fibrosis (upregulated Col1a1, Acta2, Tgfβ, Timp1). Autofluorescence analyses revealed prominent vitamin A accumulation in hepatocytes rather than HSC in HFC-fed mice. Palmitic acid exposure increased Lrat mRNA levels in primary rat hepatocytes and promoted retinyl palmitate accumulation when co-treated with retinol, which was not detected for similarly-treated primary rat HSCs.
Conclusion
NAFLD leads to cell type-specific rearrangements in retinol metabolism leading to vitamin A accumulation in hepatocytes. This may promote disease progression and/or affect therapeutic approaches targeting nuclear receptors.
中文翻译:

NAFLD 小鼠肝脏维生素 A 代谢受损,导致肝细胞中维生素 A 积累。
背景与目标
全身视黄醇(维生素 A)稳态由肝脏控制,涉及肝细胞和肝星状细胞 (HSC) 之间的密切合作。视黄醇代谢的遗传变异( PNPLA3和HSD17B13 )与非酒精性脂肪性肝病 (NAFLD) 和疾病进展相关。然而,关于 NAFLD 中肝脏维生素 A 代谢的机制细节知之甚少,它可能影响碳水化合物和脂质代谢、炎症、氧化应激以及纤维化和癌症的发展,例如 NAFLD 的所有危险因素。
方法
在这里,我们分析了 2 个 NAFLD 小鼠模型中的维生素 A 代谢;喂养高脂肪、高胆固醇 (HFC) 饮食的小鼠和饲喂瘦素ob突变体 ( ob/ob ) 的小鼠。
结果
在两种 NAFLD 小鼠模型中,肝脏视黄醇和视黄醇结合蛋白 4 (RBP4) 水平均显着降低。相比之下,这些小鼠的肝脏棕榈酸视黄酯水平(维生素 A 储存形式)显着升高。转录组分析揭示了肝脏维生素 A 代谢的高动力状态,脂肪肝中视黄醇储存和代谢增强( Lrat、Dgat1、Pnpla3 、 Raldh和 RAR/RXR 靶基因上调),同时诱导肝脏炎症( Cd68 、 Tnfα上调) 、 Nos2 、 Il1β、Il-6 )和纤维化(上调Col1a1 、 Acta2 、 Tgfβ 、 Timp1 )。自发荧光分析显示,在 HFC 喂养的小鼠中,维生素 A 明显积累在肝细胞中,而不是 HSC 中。当与视黄醇共同处理时,棕榈酸暴露增加了原代大鼠肝细胞中的Lrat mRNA 水平,并促进了棕榈酸视黄酯的积累,而在经过类似处理的原代大鼠 HSC 中未检测到这一点。
结论
NAFLD 会导致视黄醇代谢中细胞类型特异性重排,导致维生素 A 在肝细胞中积聚。这可能会促进疾病进展和/或影响针对核受体的治疗方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号