Cellular and Molecular Gastroenterology and Hepatology ( IF 7.1 ) Pub Date : 2020-06-29 , DOI: 10.1016/j.jcmgh.2020.06.009 Yin-Xin Zhu 1 , Chi Han Li 1 , Guolin Li 2 , Huiyi Feng 1 , Tian Xia 1 , Chi Hin Wong 1 , Frederic Khe Cheong Fung 1 , Joanna Hung-Man Tong 3 , Ka-Fai To 3 , Rufu Chen 4 , Yangchao Chen 5
|
Background & Aims
Gemcitabine resistance is rapidly acquired by pancreatic ductal adenocarcinoma (PDAC) patients. Novel approaches that predict the gemcitabine response of patients and enhance gemcitabine chemosensitivity are important to improve patient survival. We aimed to identify genes as novel biomarkers to predict the gemcitabine response and the therapeutic targets to attenuate chemoresistance in PDAC cells.
Methods
Genome-wide RNA interference screening was conducted to identify genes that regulated gemcitabine chemoresistance. A cell proliferation assay and a tumor formation assay were conducted to study the role of lethal giant larvae homolog 1 (LLGL1) in gemcitabine chemoresistance. Levels of LLGL1 and its regulating targets were measured by immunohistochemical staining in tumor tissues obtained from patients who received gemcitabine as a single therapeutic agent. A gene-expression microarray was conducted to identify the targets regulated by LLGL1.
Results
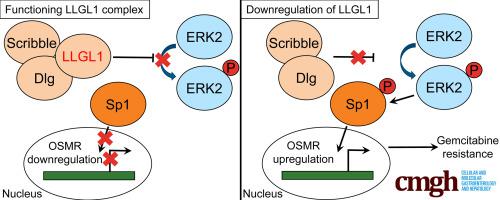
Silencing of LLGL1 markedly reduced the gemcitabine chemosensitivity in PDAC cells. Patients had significantly shorter survival (6 months) if they bore tumors expressing low LLGL1 level than tumors with high LLGL1 level (20 months) (hazard ratio, 0.1567; 95% CI, 0.05966–0.4117). Loss of LLGL1 promoted cytokine receptor oncostatin M receptor (OSMR) expression in PDAC cells that led to gemcitabine resistance, while knockdown of OSMR effectively rescued the chemoresistance phenotype. The LLGL1-OSMR regulatory pathway showed great clinical importance because low LLGL1 and high OSMR expressions were observed frequently in PDAC tissues. Silencing of LLGL1 induced phosphorylation of extracellular signal-regulated kinase 2 and specificity protein 1 (Sp1), promoted Sp1 (pThr453) binding at the OSMR promoter, and enhanced OSMR transcription.
Conclusions
LLGL1 possessed a tumor-suppressor role as an inhibitor of chemoresistance by regulating OSMR–extracellular signal-regulated kinase 2/Sp1 signaling. The data sets generated and analyzed during the current study are available in the Gene Expression Omnibus repository (ID: GSE64681).
中文翻译:

LLGL1 通过调节胰管腺癌中的 ERK-SP1-OSMR 通路来调节吉西他滨耐药性。
背景与目标
胰腺导管腺癌 (PDAC) 患者很快就会产生吉西他滨耐药性。预测患者吉西他滨反应并增强吉西他滨化疗敏感性的新方法对于提高患者生存率非常重要。我们的目的是鉴定基因作为新的生物标志物来预测吉西他滨反应和减轻 PDAC 细胞化疗耐药性的治疗靶点。
方法
进行全基因组 RNA 干扰筛选,以确定调节吉西他滨化疗耐药性的基因。通过细胞增殖测定和肿瘤形成测定来研究致死性巨型幼虫同源物 1 (LLGL1) 在吉西他滨化疗耐药中的作用。通过免疫组织化学染色测量从接受吉西他滨作为单一治疗药物的患者获得的肿瘤组织中的 LLGL1 及其调节靶点的水平。通过基因表达微阵列来鉴定 LLGL1 调节的靶标。
结果
LLGL1 的沉默显着降低了 PDAC 细胞中吉西他滨的化疗敏感性。如果患者患有表达低 LLGL1 水平的肿瘤,那么患者的生存期(6 个月)明显短于具有高 LLGL1 水平的肿瘤(20 个月)(风险比,0.1567;95% CI,0.05966–0.4117)。 LLGL1 的缺失促进了 PDAC 细胞中细胞因子受体制瘤素 M 受体 (OSMR) 的表达,从而导致吉西他滨耐药,而 OSMR 的敲低则有效地挽救了化疗耐药表型。 LLGL1-OSMR 调控通路显示出巨大的临床重要性,因为在 PDAC 组织中经常观察到低 LLGL1 和高 OSMR 表达。 LLGL1 的沉默会诱导细胞外信号调节激酶 2 和特异性蛋白 1 (Sp1) 的磷酸化,促进 Sp1 (pThr453) 在 OSMR 启动子上的结合,并增强 OSMR 转录。
结论
LLGL1 通过调节 OSMR——细胞外信号调节激酶 2/Sp1 信号传导,具有作为化疗耐药抑制剂的肿瘤抑制作用。当前研究期间生成和分析的数据集可在基因表达综合存储库(ID:GSE64681)中获取。











































 京公网安备 11010802027423号
京公网安备 11010802027423号