当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydrogenative Depolymerization of Nylons
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-07-24 , DOI: 10.1021/jacs.0c05675 Amit Kumar , Niklas von Wolff 1 , Michael Rauch , You-Quan Zou , Guy Shmul , Yehoshoa Ben-David , Gregory Leitus , Liat Avram , David Milstein
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-07-24 , DOI: 10.1021/jacs.0c05675 Amit Kumar , Niklas von Wolff 1 , Michael Rauch , You-Quan Zou , Guy Shmul , Yehoshoa Ben-David , Gregory Leitus , Liat Avram , David Milstein
Affiliation
|
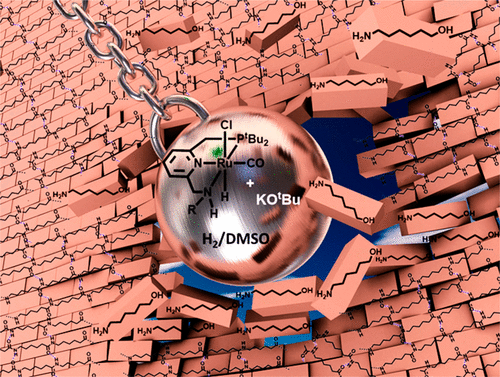
The widespread crisis of plastic pollution demands discovery of new and sustainable approaches to degrade robust plastics such as nylons. Using a green and sustainable approach based on hydrogenation, in the presence of a ruthenium pincer catalyst at 150 °C and 70 bar H2, we report here the first example of hydrogenative depolymerization of conventional, widely used nylons and polyamides, in general. Under the same catalytic conditions, we also demonstrate the hydrogenation of a polyurethane to produce diol, diamine, and methanol. Additionally, we demonstrate an example where monomers (and oligomers) obtained from the hydrogenation process can be dehydrogenated back to a poly(oligo)amide of approximately similar molecular weight, thus completing a closed loop cycle for recycling of polyamides. Based on the experimental and density functional theory studies, we propose a catalytic cycle for the process that is facilitated by metal–ligand cooperativity. Overall, this unprecedented transformation, albeit at the proof of concept level, offers a new approach toward a cleaner route to recycling nylons.
中文翻译:

尼龙的氢化解聚
塑料污染的普遍危机要求发现新的和可持续的方法来降解尼龙等坚固的塑料。使用基于氢化的绿色和可持续方法,在 150 °C 和 70 bar H2 的钌钳催化剂的存在下,我们在这里报告了常规、广泛使用的尼龙和聚酰胺的氢化解聚的第一个例子。在相同的催化条件下,我们还演示了聚氨酯加氢生成二醇、二胺和甲醇。此外,我们还展示了一个例子,其中从氢化过程中获得的单体(和低聚物)可以脱氢回到分子量大致相似的聚(低聚)酰胺,从而完成聚酰胺回收的闭环循环。基于实验和密度泛函理论研究,我们提出了由金属-配体协同促进的过程的催化循环。总体而言,这种前所未有的转变,尽管是在概念验证层面,为更清洁的尼龙回收途径提供了一种新方法。
更新日期:2020-07-24
中文翻译:

尼龙的氢化解聚
塑料污染的普遍危机要求发现新的和可持续的方法来降解尼龙等坚固的塑料。使用基于氢化的绿色和可持续方法,在 150 °C 和 70 bar H2 的钌钳催化剂的存在下,我们在这里报告了常规、广泛使用的尼龙和聚酰胺的氢化解聚的第一个例子。在相同的催化条件下,我们还演示了聚氨酯加氢生成二醇、二胺和甲醇。此外,我们还展示了一个例子,其中从氢化过程中获得的单体(和低聚物)可以脱氢回到分子量大致相似的聚(低聚)酰胺,从而完成聚酰胺回收的闭环循环。基于实验和密度泛函理论研究,我们提出了由金属-配体协同促进的过程的催化循环。总体而言,这种前所未有的转变,尽管是在概念验证层面,为更清洁的尼龙回收途径提供了一种新方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号