当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
High-Affinity Binding of LDL Receptor-Related Protein 1 to Matrix Metalloprotease 1 Requires Protease:Inhibitor Complex Formation.
Biochemistry ( IF 2.9 ) Pub Date : 2020-07-23 , DOI: 10.1021/acs.biochem.0c00442 Allison L Arai , Mary Migliorini , Dianaly T Au , Elizabeth Hahn-Dantona , David Peeney 1 , William G Stetler-Stevenson 1 , Selen C Muratoglu , Dudley K Strickland
Biochemistry ( IF 2.9 ) Pub Date : 2020-07-23 , DOI: 10.1021/acs.biochem.0c00442 Allison L Arai , Mary Migliorini , Dianaly T Au , Elizabeth Hahn-Dantona , David Peeney 1 , William G Stetler-Stevenson 1 , Selen C Muratoglu , Dudley K Strickland
Affiliation
|
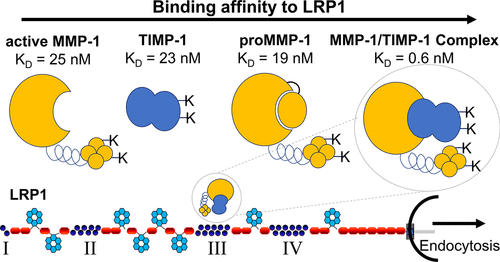
Matrix metalloprotease (MMP) activation contributes to the degradation of the extracellular matrix (ECM), resulting in a multitude of pathologies. Low-density lipoprotein receptor-related protein 1 (LRP1) is a multifaceted endocytic and signaling receptor that is responsible for internalization and lysosomal degradation of diverse proteases, protease inhibitors, and lipoproteins along with numerous other proteins. In this study, we identified MMP-1 as a novel LRP1 ligand. Binding studies employing surface plasmon resonance revealed that both proMMP-1 and active MMP-1 bind to purified LRP1 with equilibrium dissociation constants (KD) of 19 and 25 nM, respectively. We observed that human aortic smooth muscle cells readily internalize and degrade 125I-labeled proMMP-1 in an LRP1-mediated process. Our binding data also revealed that all tissue inhibitors of metalloproteases (TIMPs) bind to LRP1 with KD values ranging from 23 to 33 nM. Interestingly, the MMP-1/TIMP-1 complex bound to LRP1 with an affinity (KD = 0.6 nM) that was 30-fold higher than that of either component alone, revealing that LRP1 prefers the protease:inhibitor complex as a ligand. Of note, modification of lysine residues on either proMMP-1 or TIMP-1 ablated the ability of the MMP-1/TIMP-1 complex to bind to LRP1. LRP1’s preferential binding to enzyme:inhibitor complexes was further supported by the higher binding affinity for proMMP-9/TIMP-1 complexes than for either of these two components alone. LRP1 has four clusters of ligand-binding repeats, and MMP-1, TIMP-1, and MMP-1/TIMP-1 complexes bound to cluster III most avidly. Our results reveal an important role for LRP1 in controlling ECM homeostasis by regulating MMP-1 and MMP-9 levels.
中文翻译:

LDL 受体相关蛋白 1 与基质金属蛋白酶 1 的高亲和力结合需要蛋白酶:抑制剂复合物的形成。
基质金属蛋白酶 (MMP) 激活有助于细胞外基质 (ECM) 的降解,从而导致多种病理。低密度脂蛋白受体相关蛋白 1 (LRP1) 是一种多方面的内吞和信号受体,负责多种蛋白酶、蛋白酶抑制剂和脂蛋白以及许多其他蛋白质的内化和溶酶体降解。在这项研究中,我们将 MMP-1 鉴定为一种新型 LRP1 配体。采用表面等离子共振的结合研究表明, proMMP -1 和活性 MMP-1 均与纯化的LRP1结合,平衡解离常数 (KD ) 分别为 19 和 25 nM。我们观察到人主动脉平滑肌细胞很容易内化和降解125在 LRP1 介导的过程中,我标记了 proMMP-1。我们的结合数据还显示,所有金属蛋白酶组织抑制剂 (TIMP) 均与 LRP1 结合,K D值范围为 23 至 33 nM。有趣的是,MMP-1/TIMP-1 复合物与 LRP1 具有亲和力(K D= 0.6 nM),比单独的任一组分高 30 倍,表明 LRP1 更喜欢蛋白酶:抑制剂复合物作为配体。值得注意的是,proMMP-1 或 TIMP-1 上赖氨酸残基的修饰消除了 MMP-1/TIMP-1 复合物与 LRP1 结合的能力。LRP1 与酶:抑制剂复合物的优先结合进一步得到了对 proMMP-9/TIMP-1 复合物的更高结合亲和力的支持。LRP1 有四个配体结合重复簇,MMP-1、TIMP-1 和 MMP-1/TIMP-1 复合物与簇 III 最紧密结合。我们的结果揭示了 LRP1 通过调节 MMP-1 和 MMP-9 水平在控制 ECM 稳态中的重要作用。
更新日期:2020-08-18
中文翻译:

LDL 受体相关蛋白 1 与基质金属蛋白酶 1 的高亲和力结合需要蛋白酶:抑制剂复合物的形成。
基质金属蛋白酶 (MMP) 激活有助于细胞外基质 (ECM) 的降解,从而导致多种病理。低密度脂蛋白受体相关蛋白 1 (LRP1) 是一种多方面的内吞和信号受体,负责多种蛋白酶、蛋白酶抑制剂和脂蛋白以及许多其他蛋白质的内化和溶酶体降解。在这项研究中,我们将 MMP-1 鉴定为一种新型 LRP1 配体。采用表面等离子共振的结合研究表明, proMMP -1 和活性 MMP-1 均与纯化的LRP1结合,平衡解离常数 (KD ) 分别为 19 和 25 nM。我们观察到人主动脉平滑肌细胞很容易内化和降解125在 LRP1 介导的过程中,我标记了 proMMP-1。我们的结合数据还显示,所有金属蛋白酶组织抑制剂 (TIMP) 均与 LRP1 结合,K D值范围为 23 至 33 nM。有趣的是,MMP-1/TIMP-1 复合物与 LRP1 具有亲和力(K D= 0.6 nM),比单独的任一组分高 30 倍,表明 LRP1 更喜欢蛋白酶:抑制剂复合物作为配体。值得注意的是,proMMP-1 或 TIMP-1 上赖氨酸残基的修饰消除了 MMP-1/TIMP-1 复合物与 LRP1 结合的能力。LRP1 与酶:抑制剂复合物的优先结合进一步得到了对 proMMP-9/TIMP-1 复合物的更高结合亲和力的支持。LRP1 有四个配体结合重复簇,MMP-1、TIMP-1 和 MMP-1/TIMP-1 复合物与簇 III 最紧密结合。我们的结果揭示了 LRP1 通过调节 MMP-1 和 MMP-9 水平在控制 ECM 稳态中的重要作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号