当前位置:
X-MOL 学术
›
Acta Cryst. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, crystal structure and docking studies of tetracyclic 10-iodo-1,2-dihydroisoquinolino[2,1-b][1,2,4]benzothiadiazine 12,12-dioxide and its precursors.
Acta Crystallographica Section C ( IF 0.7 ) Pub Date : 2020-07-24 , DOI: 10.1107/s2053229620009626 Sherif O Kolade 1 , Josephat U Izunobi 1 , Eric C Hosten 2 , Idris A Olasupo 1 , Adeniyi S Ogunlaja 2 , Oluwole B Familoni 1
Acta Crystallographica Section C ( IF 0.7 ) Pub Date : 2020-07-24 , DOI: 10.1107/s2053229620009626 Sherif O Kolade 1 , Josephat U Izunobi 1 , Eric C Hosten 2 , Idris A Olasupo 1 , Adeniyi S Ogunlaja 2 , Oluwole B Familoni 1
Affiliation
|
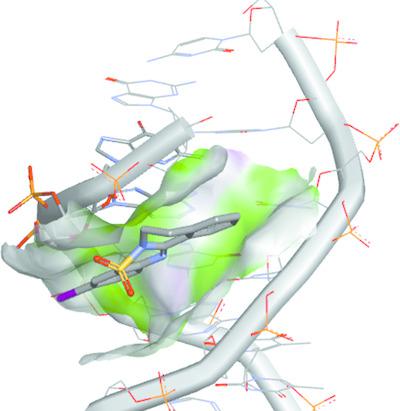
The title compound, 10‐iodo‐1,2‐dihydroisoquinolino[2,1‐b][1,2,4]benzothiadiazine 12,12‐dioxide, C15H11IN2O2S (8), was synthesized via the metal‐free intramolecular N‐iodosuccinimide (NIS)‐mediated radical oxidative sp3‐C—H aminative cyclization of 2‐(2′‐aminobenzenesulfonyl)‐1,3,4‐trihydroisoquinoline, C15H16N2O2S (7). The amino adduct 7 was prepared via a two‐step reaction, starting from the condensation of 2‐nitrobenzenesulfonyl chloride (4) with 1,2,3,4‐tetrahydroisoquinoline (5), to afford 2‐(2′‐nitrobenzenesulfonyl)‐1,3,4‐trihydroisoquinoline, C15H14N2O4S (6), in 82% yield. The catalytic hydrogenation of 6 with hydrogen gas, in the presence of 10% palladium‐on‐charcoal catalyst, furnished 7. Products 6–8 were characterized by their melting points, IR and NMR (1H and 13C) spectroscopy, and single‐crystal X‐ray diffraction. The three compounds crystallized in the monoclinic space group, with 7 exhibiting classical intramolecular hydrogen bonds of 2.16 and 2.26 Å. All three crystal structures exhibit centrosymmetric pairs of intermolecular C—H…π(ring) and/or π–π stacking interactions. The docking studies of molecules 6, 7 and 8 with deoxyribonucleic acid (PDB id: 1ZEW) revealed minor‐groove binding behaviours without intercalation, with 7 presenting the most favourable global energy of the three molecules. Nonetheless, molecule 8 interacted strongly with the DNA macromolecule, with an attractive van der Waals energy of −15.53 kcal mol−1.
中文翻译:

四环10-碘-1,2-二氢异喹啉[2,1-b] [1,2,4]苯并噻二嗪12,12-二氧化物及其前体的合成,晶体结构和对接研究。
的标题化合物,10碘-1,2- dihydroisoquinolino [2,1- b ] [1,2,4]苯并噻二嗪12,12二氧化物,C 15 ħ 11 IN 2 ö 2 S(8),合成通过分子内无金属的N-碘琥珀酰亚胺(NIS)介导的自由基氧化sp 3 -C-H环化2-(2'-氨基苯磺酰基)-1,3,4-三氢异喹啉,C 15 H 16 N 2 O 2 S (7)。的氨基加合物7制备通过从2-硝基苯磺酰氯(4)与1,2,3,4-四氢异喹啉(5)缩合开始的两步反应,得到2-(2'-硝基苯磺酰基)-1,3,4-三氢异喹啉C 15 H 14 N 2 O 4 S(6),产率82%。在10%钯/木炭催化剂存在下,用氢气催化加氢6,得到7。产品6 - 8进行了表征可以通过熔点,IR和NMR(1 H和13C)光谱学和单晶X射线衍射。这三种化合物在单斜晶空间群中结晶,其中7种表现出2.16和2.26Å的经典分子内氢键。所有这三种晶体结构均表现出中心对称的分子间CH-π(环)对和/或π-π堆积相互作用。分子的对接研究6,7和8与脱氧核糖核酸(PDB ID:1ZEW)揭示小沟结合行为,而不插层,用7呈现三个分子的最有利的全局能量。尽管如此,分子8与DNA大分子强烈相互作用,其范德华力为-15.53 kcal mol -1。
更新日期:2020-07-24
中文翻译:

四环10-碘-1,2-二氢异喹啉[2,1-b] [1,2,4]苯并噻二嗪12,12-二氧化物及其前体的合成,晶体结构和对接研究。
的标题化合物,10碘-1,2- dihydroisoquinolino [2,1- b ] [1,2,4]苯并噻二嗪12,12二氧化物,C 15 ħ 11 IN 2 ö 2 S(8),合成通过分子内无金属的N-碘琥珀酰亚胺(NIS)介导的自由基氧化sp 3 -C-H环化2-(2'-氨基苯磺酰基)-1,3,4-三氢异喹啉,C 15 H 16 N 2 O 2 S (7)。的氨基加合物7制备通过从2-硝基苯磺酰氯(4)与1,2,3,4-四氢异喹啉(5)缩合开始的两步反应,得到2-(2'-硝基苯磺酰基)-1,3,4-三氢异喹啉C 15 H 14 N 2 O 4 S(6),产率82%。在10%钯/木炭催化剂存在下,用氢气催化加氢6,得到7。产品6 - 8进行了表征可以通过熔点,IR和NMR(1 H和13C)光谱学和单晶X射线衍射。这三种化合物在单斜晶空间群中结晶,其中7种表现出2.16和2.26Å的经典分子内氢键。所有这三种晶体结构均表现出中心对称的分子间CH-π(环)对和/或π-π堆积相互作用。分子的对接研究6,7和8与脱氧核糖核酸(PDB ID:1ZEW)揭示小沟结合行为,而不插层,用7呈现三个分子的最有利的全局能量。尽管如此,分子8与DNA大分子强烈相互作用,其范德华力为-15.53 kcal mol -1。











































 京公网安备 11010802027423号
京公网安备 11010802027423号