当前位置:
X-MOL 学术
›
Acta Cryst. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The different modes of chiral [1,2,3]triazolo[5,1-b][1,3,4]thiadiazines: crystal packing, conformation investigation and cellular activity.
Acta Crystallographica Section C ( IF 0.7 ) Pub Date : 2020-07-23 , DOI: 10.1107/s2053229620009328 Konstantin L'vovich Obydennov 1 , Tatiana Andreevna Kalinina 1 , Olga Alexandrovna Vysokova 1 , Pavel Alexandrovich Slepukhin 2 , Varvara Alexandrovna Pozdina 3 , Maria Valer'evna Ulitko 3 , Tatiana Vladimirovna Glukhareva 1
Acta Crystallographica Section C ( IF 0.7 ) Pub Date : 2020-07-23 , DOI: 10.1107/s2053229620009328 Konstantin L'vovich Obydennov 1 , Tatiana Andreevna Kalinina 1 , Olga Alexandrovna Vysokova 1 , Pavel Alexandrovich Slepukhin 2 , Varvara Alexandrovna Pozdina 3 , Maria Valer'evna Ulitko 3 , Tatiana Vladimirovna Glukhareva 1
Affiliation
|
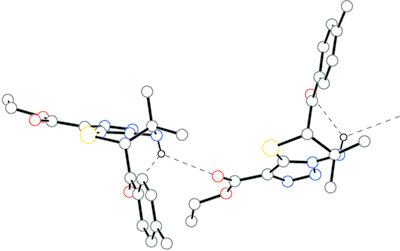
The crystal structures of four new chiral [1,2,3]triazolo[5,1‐b][1,3,4]thiadiazines are described, namely, ethyl 5′‐benzoyl‐5′H,7′H‐spiro[cyclohexane‐1,6′‐[1,2,3]triazolo[5,1‐b][1,3,4]thiadiazine]‐3′‐carboxylate, C19H22N4O3S, ethyl 5′‐(4‐methoxybenzoyl)‐5′H,7′H‐spiro[cyclohexane‐1,6′‐[1,2,3]triazolo[5,1‐b][1,3,4]thiadiazine]‐3′‐carboxylate, C20H24N4O4S, ethyl 6,6‐dimethyl‐5‐(4‐methylbenzoyl)‐6,7‐dihydro‐5H‐[1,2,3]triazolo[5,1‐b][1,3,4]thiadiazine‐3‐carboxylate, C17H20N4O3S, and ethyl 5‐benzoyl‐6‐(4‐methoxyphenyl)‐6,7‐dihydro‐5H‐[1,2,3]triazolo[5,1‐b][1,3,4]thiadiazine‐3‐carboxylate, C21H20N4O4S. The crystallographic data and cell activities of these four compounds and of the structures of three previously reported similar compounds, namely, ethyl 5′‐(4‐methylbenzoyl)‐5′H,7′H‐spiro[cyclopentane‐1,6′‐[1,2,3]triazolo[5,1‐b][1,3,4]thiadiazine]‐3′‐carboxylate, C19H22N4O3S, ethyl 5′‐(4‐methoxybenzoyl)‐5′H,7′H‐spiro[cyclopentane‐1,6′‐[1,2,3]triazolo[5,1‐b][1,3,4]thiadiazine]‐3′‐carboxylate, C19H22N4O4S, and ethyl 6‐methyl‐5‐(4‐methylbenzoyl)‐6‐phenyl‐6,7‐dihydro‐5H‐[1,2,3]triazolo[5,1‐b][1,3,4]thiadiazine‐3‐carboxylate, C22H22N4O3S, are contrasted and compared. For both crystallization and an MTT assay, racemic mixtures of the corresponding [1,2,3]triazolo[5,1‐b][1,3,4]thiadiazines were used. The main manner of molecular packing in these compounds is the organization of either enantiomeric pairs or dimers. In both cases, the formation of two three‐centre hydrogen bonds can be detected resulting from intramolecular N—H…O and intermolecular N—H…O or N—H…N interactions. Molecules of different enantiomeric forms can also form chains through N—H…O hydrogen bonds or form layers between which only weak hydrophobic contacts exist. Unlike other [1,2,3]triazolo[5,1‐b][1,3,4]thiadiazines, ethyl 5′‐benzoyl‐5′H,7′H‐spiro[cyclohexane‐1,6′‐[1,2,3]triazolo[5,1‐b][1,3,4]thiadiazine]‐3′‐carboxylate contains molecules of only the (R)‐enantiomer; moreover, the N—H group does not participate in any significant intermolecular interactions. Molecular mechanics methods (force field OPLS3e) and the DFT B3LYP/6‐31G+(d,p) method show that the compound forming enantiomeric pairs via weak N—H…N hydrogen bonds is subject to greater distortion of the geometry under the influence of the intermolecular interactions in the crystal. For intramolecular N—H…O and S…O interactions, an analysis of the noncovalent interactions (NCIs) was carried out. The cellular activities of the compounds were tested by evaluating their antiproliferative effect against two normal human cell lines and two cancer cell lines in terms of half‐maximum inhibitory concentration (IC50). Some derivatives have been found to be very effective in inhibiting the growth of Hela cells at nanomolar and submicromolar concentrations with minimal cytotoxicity in relation to normal cells.
中文翻译:

手性[1,2,3]三唑[5,1-b] [1,3,4]噻二嗪的不同模式:晶体堆积,构象研究和细胞活性。
四个新的手性[1,2,3]三唑并[5,1-晶体结构b ] [1,3,4]噻二嗪中描述,即,乙基5'-苯甲酰基-5' ħ,7' ħ -螺[环己烷-1,6'-[1,2,3]三唑[5,1- b ] [1,3,4]噻二嗪] -3'-羧酸盐,C 19 H 22 N 4 O 3 S,乙基5 ' - (4-甲氧基苯甲酰基)-5' ħ,7' ħ -螺[环己烷-1,6' - [1,2,3]三唑并[5,1- b ] [1,3,4]噻二嗪] - 3'-羧酸盐,C 20 H 24 N 4 O 4 S,乙基6,6-二甲基-5-(4-甲基苯甲酰基)-6,7-二氢-5 H- [1,2,3]三唑[5, 1‐b ] [1,3,4]噻二嗪-3-羧酸乙酯,C 17 ħ 20 Ñ 4 ø 3 S,和5-苯甲酰基-6-(4-甲氧基苯基)-6,7-二氢-5- ħ - [1- 1,2,3]三唑并[5,1- b ] [1,3,4]噻二嗪-3-羧酸乙酯,C 21 ħ 20 ñ 4 ø 4 S的晶体学数据和这四种化合物的细胞活性和结构的三个先前报道相似的化合物,即,乙基-5' - (4-甲基苯甲酰基)-5' ħ,7' ħ -螺[环戊烷-1,6' - [1,2,3]三唑并[5,1- b ] [1,3,4]噻二嗪] -3'-羧酸盐,C 19 H 22Ñ 4 ø 3 S,乙基-5' - (4-甲氧基苯甲酰基)-5' ħ,7' ħ -螺[环戊烷-1,6' - [1,2,3]三唑并[5,1- b ] [1 1,3,4]噻二嗪] -3'-羧酸酯,C 19 ħ 22 ñ 4 Ò 4 S,和乙基-6-甲基-5-(4-甲基苯甲酰基)-6-苯基-6,7-二氢-5- ħ - [1,2,3]三唑并[5,1- b ] [1,3,4]噻二嗪-3-羧酸乙酯,C 22 ħ 22 ñ 4 ø 3 S,进行对比和比较。对于结晶和MTT分析,都需要相应的[1,2,3]三唑[5,1- b ]的外消旋混合物使用[1,3,4]噻二嗪。这些化合物中分子堆积的主要方式是对映体对或二聚体的组织。在这两种情况下,均可检测到分子内NH-O和分子间NH-O或NH-N相互作用形成的两个三个中心氢键。不同对映体形式的分子也可以通过NH…O氢键形成链,或形成仅存在弱疏水接触的层。不像其他的[1,2,3]三唑并[5,1- b ] [1,3,4]噻二嗪,乙基5'-苯甲酰基-5' ħ,7' ħ -螺[环己烷-1,6' - [ 1,2,3]三唑并[5,1– b ] [1,3,4]噻二嗪] -3′-羧酸盐仅包含(R对映体; 此外,NH基团不参与任何重要的分子间相互作用。分子力学方法(力场OPLS3e)和DFT B3LYP / 6-31G +(d,p)方法显示,通过弱NH -N氢键形成对映体的化合物在下列条件下受到更大的几何变形影响:晶体中的分子间相互作用。对于分子内NH-O和S-O相互作用,进行了非共价相互作用(NCI)的分析。通过评估化合物对两种正常人细胞系和两种癌细胞系的半数最大抑制浓度的抗增殖作用来测试其细胞活性(IC 50)。已经发现一些衍生物在纳摩尔和亚微摩尔浓度下对抑制Hela细胞的生长非常有效,相对于正常细胞而言具有最小的细胞毒性。
更新日期:2020-07-23
中文翻译:

手性[1,2,3]三唑[5,1-b] [1,3,4]噻二嗪的不同模式:晶体堆积,构象研究和细胞活性。
四个新的手性[1,2,3]三唑并[5,1-晶体结构b ] [1,3,4]噻二嗪中描述,即,乙基5'-苯甲酰基-5' ħ,7' ħ -螺[环己烷-1,6'-[1,2,3]三唑[5,1- b ] [1,3,4]噻二嗪] -3'-羧酸盐,C 19 H 22 N 4 O 3 S,乙基5 ' - (4-甲氧基苯甲酰基)-5' ħ,7' ħ -螺[环己烷-1,6' - [1,2,3]三唑并[5,1- b ] [1,3,4]噻二嗪] - 3'-羧酸盐,C 20 H 24 N 4 O 4 S,乙基6,6-二甲基-5-(4-甲基苯甲酰基)-6,7-二氢-5 H- [1,2,3]三唑[5, 1‐b ] [1,3,4]噻二嗪-3-羧酸乙酯,C 17 ħ 20 Ñ 4 ø 3 S,和5-苯甲酰基-6-(4-甲氧基苯基)-6,7-二氢-5- ħ - [1- 1,2,3]三唑并[5,1- b ] [1,3,4]噻二嗪-3-羧酸乙酯,C 21 ħ 20 ñ 4 ø 4 S的晶体学数据和这四种化合物的细胞活性和结构的三个先前报道相似的化合物,即,乙基-5' - (4-甲基苯甲酰基)-5' ħ,7' ħ -螺[环戊烷-1,6' - [1,2,3]三唑并[5,1- b ] [1,3,4]噻二嗪] -3'-羧酸盐,C 19 H 22Ñ 4 ø 3 S,乙基-5' - (4-甲氧基苯甲酰基)-5' ħ,7' ħ -螺[环戊烷-1,6' - [1,2,3]三唑并[5,1- b ] [1 1,3,4]噻二嗪] -3'-羧酸酯,C 19 ħ 22 ñ 4 Ò 4 S,和乙基-6-甲基-5-(4-甲基苯甲酰基)-6-苯基-6,7-二氢-5- ħ - [1,2,3]三唑并[5,1- b ] [1,3,4]噻二嗪-3-羧酸乙酯,C 22 ħ 22 ñ 4 ø 3 S,进行对比和比较。对于结晶和MTT分析,都需要相应的[1,2,3]三唑[5,1- b ]的外消旋混合物使用[1,3,4]噻二嗪。这些化合物中分子堆积的主要方式是对映体对或二聚体的组织。在这两种情况下,均可检测到分子内NH-O和分子间NH-O或NH-N相互作用形成的两个三个中心氢键。不同对映体形式的分子也可以通过NH…O氢键形成链,或形成仅存在弱疏水接触的层。不像其他的[1,2,3]三唑并[5,1- b ] [1,3,4]噻二嗪,乙基5'-苯甲酰基-5' ħ,7' ħ -螺[环己烷-1,6' - [ 1,2,3]三唑并[5,1– b ] [1,3,4]噻二嗪] -3′-羧酸盐仅包含(R对映体; 此外,NH基团不参与任何重要的分子间相互作用。分子力学方法(力场OPLS3e)和DFT B3LYP / 6-31G +(d,p)方法显示,通过弱NH -N氢键形成对映体的化合物在下列条件下受到更大的几何变形影响:晶体中的分子间相互作用。对于分子内NH-O和S-O相互作用,进行了非共价相互作用(NCI)的分析。通过评估化合物对两种正常人细胞系和两种癌细胞系的半数最大抑制浓度的抗增殖作用来测试其细胞活性(IC 50)。已经发现一些衍生物在纳摩尔和亚微摩尔浓度下对抑制Hela细胞的生长非常有效,相对于正常细胞而言具有最小的细胞毒性。










































 京公网安备 11010802027423号
京公网安备 11010802027423号