当前位置:
X-MOL 学术
›
Mol. Microbiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Agl22 and Agl23 are involved in the synthesis and utilization of the lipid-linked intermediates in the glycosylation pathways of the halophilic archaeaon Haloarcula hispanica.
Molecular Microbiology ( IF 2.6 ) Pub Date : 2020-07-24 , DOI: 10.1111/mmi.14577 Hua Lu 1, 2 , Caixia Pei 1, 2 , Hui Zhou 1 , Yang Lü 1 , Yun He 3 , Yunsen Li 3 , Jing Han 4 , Hua Xiang 4 , Jerry Eichler 5 , Cheng Jin 1, 2
Molecular Microbiology ( IF 2.6 ) Pub Date : 2020-07-24 , DOI: 10.1111/mmi.14577 Hua Lu 1, 2 , Caixia Pei 1, 2 , Hui Zhou 1 , Yang Lü 1 , Yun He 3 , Yunsen Li 3 , Jing Han 4 , Hua Xiang 4 , Jerry Eichler 5 , Cheng Jin 1, 2
Affiliation
|
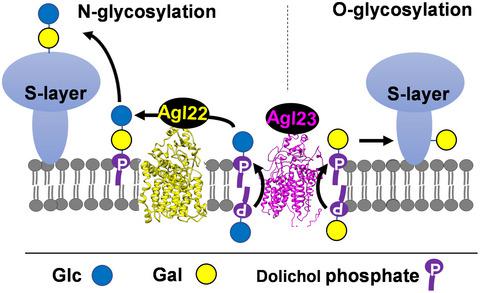
Like both eukaryotes and bacteria, archaea can decorate proteins with N‐ and O‐linked glycans. Whereas pathways and roles of N‐glycosylation have been studied in several model archaeal organisms, little is known of O‐glycosylation. To explore commonalities and variations of these two versions of glycosylation, we used Haloarcula hispanica as a model. Our previous work showed that H. hispanica S‐layer glycoproteins are modified by an N‐linked glucose‐α‐(1, 2)‐[sulfoquinovosamine‐β‐(1, 6)‐]galactose trisaccharide and an O‐linked glucose‐α‐(1, 4)‐galactose disaccharide. Here, we found that H. hispanica membrane contains C60 dolichol phosphate (DolP) as a lipid carrier for glycosylation. As revealed by bioinformatics, gene deletion and phenotype analysis, gene HAH_1571, renamed agl22, encodes a predicted glucosyltransferase that transfers glucose from glucose‐DolP onto galactose‐DolP to form the glucose‐α‐(1, 4)‐galactose‐DolP precursor of the N‐glycosylation. Gene HAH_2016, renamed agl23, encodes a putative flippase‐associated protein responsible for flipping of hexose‐DolPs across the membrane to face the exterior. Our results also suggested that the synthesis of the N‐ and O‐linked glycans onto target protein occurs on the outer surface of the cell using hexose‐DolPs as sugar donors. Deletion mutant showed that N‐ and O‐glycosylation are required for growth in the defined medium mimicking the natural habitat of H. hispanica.
中文翻译:

Agl22 和 Agl23 参与嗜盐古菌 Haloarcula hispanica 糖基化途径中脂质连接中间体的合成和利用。
像真核生物和细菌一样,古细菌可以用 N 和 O 连接的聚糖修饰蛋白质。尽管已经在几种模型古细菌生物中研究了 N-糖基化的途径和作用,但对 O-糖基化知之甚少。为了探索这两种糖基化版本的共性和变化,我们使用Haloarcula hispanica作为模型。我们之前的工作表明,H. hispanica S 层糖蛋白被 N-连接的葡萄糖-α-(1, 2)-[磺基奎诺糖胺-β-(1, 6)-]半乳糖三糖和 O-连接的葡萄糖- α-(1, 4)-半乳糖二糖。在这里,我们发现H. hispanica膜含有 C60 多萜醇磷酸盐 (DolP) 作为糖基化的脂质载体。生物信息学、基因缺失和表型分析表明,基因HAH_1571,重命名为agl22,编码预测的葡萄糖基转移酶,该酶将葡萄糖从葡萄糖-DolP 转移到半乳糖-DolP 上,形成 N-糖基化的葡萄糖-α-(1, 4)-半乳糖-DolP 前体。基因HAH_2016,更名为agl23,编码一种假定的翻转酶相关蛋白,负责翻转己糖-DolP 跨膜面向外部。我们的研究结果还表明,使用己糖-DolP 作为糖供体,N 和 O 连接聚糖在目标蛋白上的合成发生在细胞的外表面上。缺失突变体表明,在模拟H. hispanica自然栖息地的确定培养基中生长需要 N-和 O-糖基化。
更新日期:2020-07-24
中文翻译:

Agl22 和 Agl23 参与嗜盐古菌 Haloarcula hispanica 糖基化途径中脂质连接中间体的合成和利用。
像真核生物和细菌一样,古细菌可以用 N 和 O 连接的聚糖修饰蛋白质。尽管已经在几种模型古细菌生物中研究了 N-糖基化的途径和作用,但对 O-糖基化知之甚少。为了探索这两种糖基化版本的共性和变化,我们使用Haloarcula hispanica作为模型。我们之前的工作表明,H. hispanica S 层糖蛋白被 N-连接的葡萄糖-α-(1, 2)-[磺基奎诺糖胺-β-(1, 6)-]半乳糖三糖和 O-连接的葡萄糖- α-(1, 4)-半乳糖二糖。在这里,我们发现H. hispanica膜含有 C60 多萜醇磷酸盐 (DolP) 作为糖基化的脂质载体。生物信息学、基因缺失和表型分析表明,基因HAH_1571,重命名为agl22,编码预测的葡萄糖基转移酶,该酶将葡萄糖从葡萄糖-DolP 转移到半乳糖-DolP 上,形成 N-糖基化的葡萄糖-α-(1, 4)-半乳糖-DolP 前体。基因HAH_2016,更名为agl23,编码一种假定的翻转酶相关蛋白,负责翻转己糖-DolP 跨膜面向外部。我们的研究结果还表明,使用己糖-DolP 作为糖供体,N 和 O 连接聚糖在目标蛋白上的合成发生在细胞的外表面上。缺失突变体表明,在模拟H. hispanica自然栖息地的确定培养基中生长需要 N-和 O-糖基化。









































 京公网安备 11010802027423号
京公网安备 11010802027423号