当前位置:
X-MOL 学术
›
ChemPlusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Activating both Halogen and Chalcogen Bonding Interactions in Cation Radical Salts of Iodinated Tetrathiafulavalene Derivatives.
ChemPlusChem ( IF 3.0 ) Pub Date : 2020-07-24 , DOI: 10.1002/cplu.202000500 Maxime Beau 1 , Olivier Jeannin 1 , Sunhee Lee 2 , Frédéric Barrière 1 , Marc Fourmigué 1 , Ie-Rang Jeon 1
ChemPlusChem ( IF 3.0 ) Pub Date : 2020-07-24 , DOI: 10.1002/cplu.202000500 Maxime Beau 1 , Olivier Jeannin 1 , Sunhee Lee 2 , Frédéric Barrière 1 , Marc Fourmigué 1 , Ie-Rang Jeon 1
Affiliation
|
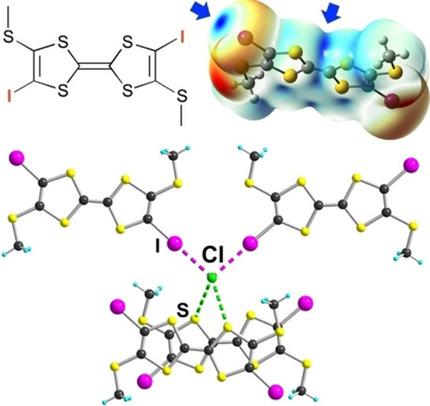
Halogen bonding (XB) interactions are investigated in cation radical salts of bis(methylthio)‐5,5’‐diiodotetrathiafulvalene (1). Electrocrystallization of 1 in the presence of Bu4NCl affords a 1 : 1 salt formulated as (E‐1)Cl. Particularly strong I⋅⋅⋅Cl− XB interactions are observed around the Cl− anion with the distances at 78 % the sum of the van der Waals radii, a consequence of the XB charge activation in the cation radical. Moreover, the Cl− environment is complemented by two extra S⋅⋅⋅Cl− chalcogen bonding (ChB) interactions, an original feature among reported halide salts of TTF derivatives. Electrostatic potential calculations on the cation radical further demonstrate the efficient activation of the S atoms of the 1,3‐dithiole rings (Vs,max=87.2 kcal/mol), as strong as with the iodine atoms (Vs,max=87.9 kcal/mol). The radical cations form weakly dimerized stacks, as confirmed by the variable‐temperature magnetic susceptibility and the weak conductivity (4.8×10−5 S cm−1).
中文翻译:

活化碘化四硫富铝富瓦烯衍生物的阳离子自由基盐中的卤素键和硫族键相互作用。
研究了双(甲硫基)-5,5'-二碘四硫富富瓦烯的阳离子自由基盐中的卤素键(XB)相互作用(1)。的电结晶1中卜存在4的NCI得到1:配制为(1盐ë - 1)氯。特别强I⋅⋅⋅Cl - XB相互作用围绕氯观察-阴离子与距离在78%的范德华半径,在阳离子基团XB电荷激活的结果的总和。此外,氯-环境是由两个额外的补充S⋅⋅⋅Cl -硫族元素键(ChB)相互作用,已报道的TTF衍生物卤化物盐的原始特征。阳离子自由基的静电势计算进一步证明了1,3-二硫环的S原子的有效活化(V s,max = 87.2 kcal / mol),与碘原子(V s,max = 87.9 )一样强。 kcal / mol)。自由基阳离子形成弱二聚化的堆叠,这由温度可变磁化率和弱电导率(4.8×10 -5 S cm -1)证实。
更新日期:2020-09-21
中文翻译:

活化碘化四硫富铝富瓦烯衍生物的阳离子自由基盐中的卤素键和硫族键相互作用。
研究了双(甲硫基)-5,5'-二碘四硫富富瓦烯的阳离子自由基盐中的卤素键(XB)相互作用(1)。的电结晶1中卜存在4的NCI得到1:配制为(1盐ë - 1)氯。特别强I⋅⋅⋅Cl - XB相互作用围绕氯观察-阴离子与距离在78%的范德华半径,在阳离子基团XB电荷激活的结果的总和。此外,氯-环境是由两个额外的补充S⋅⋅⋅Cl -硫族元素键(ChB)相互作用,已报道的TTF衍生物卤化物盐的原始特征。阳离子自由基的静电势计算进一步证明了1,3-二硫环的S原子的有效活化(V s,max = 87.2 kcal / mol),与碘原子(V s,max = 87.9 )一样强。 kcal / mol)。自由基阳离子形成弱二聚化的堆叠,这由温度可变磁化率和弱电导率(4.8×10 -5 S cm -1)证实。











































 京公网安备 11010802027423号
京公网安备 11010802027423号