Tetrahedron ( IF 2.1 ) Pub Date : 2020-07-24 , DOI: 10.1016/j.tet.2020.131437 Maciej E. Domaradzki , Xiaochen Liu , Jiye Ong , Gyeongah Yu , Gan Zhang , Ariel Simantov , Eliyahu Perl , Yu Chen
|
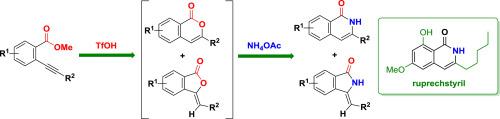
A triflic acid (TfOH) mediated sequential cyclization of ortho-alkynylarylesters and ammonium acetate (NH4OAc) was reported. The reaction took place via a Brønsted acid-mediated intramolecular cyclization of ortho-alkynylarylesters followed by an ammonium acetate participated substitution reaction, forming isoquinolin-1-ones as the major products. Different from most of the known synthetic methods of isoquinolin-1-ones, no metal catalyst was required in the reported reaction. The regioisomers – isoindolin-1-ones were obtained together with isoquinolin-1-ones in a few cases. The intermediate compounds – isochromen-1-ones and isobenzofuran-1-ones were also isolated. The interconversion experiments showed that the regioisomers formed during the Brønsted acid induced intramolecular cyclization of ortho-alkynylarylesters. A natural product – ruprechstyril was prepared in a moderate yield employing the new method.
中文翻译:

三氟乙酸与乙酸铵介导的邻炔基芳基酯的顺序环化
报道了三氟乙酸(TfOH)介导的邻炔基芳基酯和乙酸铵(NH 4 OAc)的顺序环化。该反应通过布朗斯台德酸介导的邻位分子内环化反应进行-炔基芳基酯接着乙酸铵参与取代反应,形成异喹啉-1-酮为主要产物。与大多数已知的异喹啉-1-酮合成方法不同,所报道的反应不需要金属催化剂。在少数情况下,获得了区域异构体–异吲哚啉-1-酮与异喹啉-1-酮。还分离了中间体化合物-异色酮-1-酮和异苯并呋喃-1-酮。相互转化实验表明,在布朗斯台德酸诱导的邻炔基芳基酯分子内环化过程中形成的区域异构体。采用这种新方法,可以以中等收率制备天然产物ruprechstyril。











































 京公网安备 11010802027423号
京公网安备 11010802027423号