Journal of Allergy and Clinical Immunology ( IF 11.4 ) Pub Date : 2020-07-24 , DOI: 10.1016/j.jaci.2020.06.035 Jonathan J Lyons 1 , Jack Chovanec 1 , Michael P O'Connell 1 , Yihui Liu 1 , Julij Šelb 2 , Roberta Zanotti 3 , Yun Bai 1 , Jiwon Kim 1 , Quang T Le 4 , Tom DiMaggio 1 , Lawrence B Schwartz 4 , Hirsh D Komarow 1 , Matija Rijavec 2 , Melody C Carter 1 , Joshua D Milner 5 , Patrizia Bonadonna 6 , Dean D Metcalfe 1 , Peter Korošec 2
|
Background
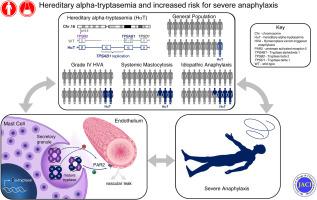
An elevated basal serum tryptase level is associated with severe systemic anaphylaxis, most notably caused by Hymenoptera envenomation. Although clonal mast cell disease is the culprit in some individuals, it does not fully explain this clinical association.
Objective
Our aim was to determine the prevalence and associated impact of tryptase genotypes on anaphylaxis in humans.
Methods
Cohorts with systemic mastocytosis (SM) and venom as well as idiopathic anaphylaxis from referral centers in Italy, Slovenia, and the United States, underwent tryptase genotyping by droplet digital PCR. Associated anaphylaxis severity (Mueller scale) was subsequently examined. Healthy volunteers and controls with nonatopic disease were recruited and tryptase was genotyped by droplet digital PCR and in silico analysis of genome sequence, respectively. The effects of pooled and recombinant human tryptases, protease activated receptor 2 agonist and antagonist peptides, and a tryptase-neutralizing mAb on human umbilical vein endothelial cell permeability were assayed using a Transwell system.
Results
Hereditary α-tryptasemia (HαT)—a genetic trait caused by increased α-tryptase–encoding Tryptase-α/β1 (TPSAB1) copy number resulting in elevated BST level—was common in healthy individuals (5.6% [n = 7 of 125]) and controls with nonatopic disease (5.3% [n = 21 of 398]). HαT was associated with grade IV venom anaphylaxis (relative risk = 2.0; P < .05) and more prevalent in both idiopathic anaphylaxis (n = 8 of 47; [17%; P = .006]) and SM (n = 10 of 82 [12.2%; P = .03]) relative to the controls. Among patients with SM, concomitant HαT was associated with increased risk for systemic anaphylaxis (relative risk = 9.5; P = .007). In vitro, protease-activated receptor-2–dependent vascular permeability was induced by pooled mature tryptases but not α- or β-tryptase homotetramers.
Conclusions
Risk for severe anaphylaxis in humans is associated with inherited differences in α-tryptase–encoding copies at TPSAB1.
中文翻译:

与 TPSAB1 编码 α-类胰蛋白酶的生殖系拷贝数增加相关的严重过敏反应的遗传风险
背景
升高的基础血清类胰蛋白酶水平与严重的全身性过敏反应有关,最显着的是由膜翅目昆虫毒液引起。尽管克隆性肥大细胞病是某些个体的罪魁祸首,但它并不能完全解释这种临床关联。
客观的
我们的目的是确定类胰蛋白酶基因型对人类过敏反应的普遍性和相关影响。
方法
来自意大利、斯洛文尼亚和美国转诊中心的系统性肥大细胞增多症 (SM) 和毒液以及特发性过敏反应队列通过液滴数字 PCR 进行类胰蛋白酶基因分型。随后检查了相关的过敏反应严重程度(穆勒量表)。招募了健康志愿者和患有非特应性疾病的对照,并分别通过液滴数字 PCR 和基因组序列的计算机分析对类胰蛋白酶进行基因分型。使用 Transwell 系统测定合并和重组人类胰蛋白酶、蛋白酶激活受体 2 激动剂和拮抗剂肽以及类胰蛋白酶中和 mAb 对人脐静脉内皮细胞通透性的影响。
结果
遗传性 α-类胰蛋白酶血症 (HαT)——一种由编码 α-类胰蛋白酶的类胰蛋白酶-α/β1 ( TPSAB1 ) 拷贝数增加导致 BST 水平升高引起的遗传特征——在健康个体中很常见(5.6% [n = 7 of 125]) ) 和非特应性疾病对照 (5.3% [n = 21 of 398])。HαT 与 IV 级毒液过敏反应(相对风险 = 2.0;P < .05)相关,并且在特发性过敏反应(n = 8 of 47;[17%; P = .006])和 SM(n = 10 of 82 [12.2%; P = .03]) 相对于对照。在 SM 患者中,伴随的 HαT 与全身性过敏反应的风险增加相关(相对风险 = 9.5;P = .007)。体外,蛋白酶激活受体 2 依赖的血管通透性是由汇集的成熟类胰蛋白酶诱导的,而不是由 α-或 β-类胰蛋白酶同四聚体诱导的。
结论
人类严重过敏反应的风险与TPSAB1 α-类胰蛋白酶编码拷贝的遗传差异有关。









































 京公网安备 11010802027423号
京公网安备 11010802027423号