Comparative Biochemistry and Physiology A: Molecular & Integrative Physiology ( IF 2.3 ) Pub Date : 2020-07-24 , DOI: 10.1016/j.cbpa.2020.110776 Van Pham Thi Ha To 1 , Marina Subramaniam 1 , Karthik Masagounder 2 , Matthew E Loewen 1
|
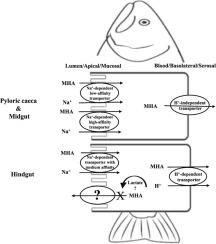
The aim of this study was to identify the unknown transport mechanism of the extensively used monocarboxylate methionine feed supplement DL-methionine hydroxyl analogue (DL-MHA) in rainbow trout intestine. Transport across the pyloric caeca (PC), midgut (MG), and hindgut (HG) regions were kinetically studied in Na+- and H+-dependent manners. Gene expression of monocarboxylate (MCTs) and sodium monocarboxylate transporters (SMCTs) were assessed. Results demonstrated that DL-MHA transport from 0.2–20 mM was Na+-dependent and obeyed Michaelis-Menten kinetics with low affinity in PC & MG in apical/basal pH of 7.7/7.7. Changes in apical/basal pH (6.0/6.0, 6.0/7.7, and 7.7/8.7) had insignificant effects on kinetics. In contrast, HG flux kinetics were only obtained in pH 7.7/8.7 or in the presence of lactate with medium affinity. Additionally, DL-MHA transport from 0 to 150 μM demonstrated the presence of a Na+-dependent high-affinity transporter in PC & MG. Conclusively, two distinct carrier-mediated DL-MHA transport mechanisms along the trout gut were found: 1) in PC & MG: apical transport was regulated by Na+-requiring systems that possibly contained low- and high-affinity transporters, and basolateral transport was primarily achieved through a H+-independent transporter; 2) in HG: uptake was apically mediated by a Na+-dependent transporter with medium affinity, and basolateral exit was largely controlled by an H+-dependent transporter. Finally, two major methionine feed supplements, DL-MHA and DL-methionine (DL-Met) were compared to understand the differences in their bioefficiency. Flux rates of DL-MHA were only about 42.2–66.0% in PC and MG compared to DL-Met, suggesting intestinal transport of DL-MHA was lower than DL-Met.
中文翻译:

DL-蛋氨酸羟基类似物沿着虹鳟鱼肠道的分段转运机制的特征以及与DL-蛋氨酸的比较。
这项研究的目的是确定虹鳟鱼肠中广泛使用的单羧酸蛋氨酸饲料补充剂DL-蛋氨酸羟基类似物(DL-MHA)的未知转运机制。以Na + -和H +依赖性方式动力学研究了跨幽门盲肠(PC),中肠(MG)和后肠(HG)区域的转运。评估了单羧酸盐(MCTs)和单羧酸钠转运蛋白(SMCTs)的基因表达。结果表明,从0.2–20 mM的DL-MHA转运为Na +-在7.7 / 7.7的根部/基部pH中,与PC和MG的亲和力较低,并且遵守Michaelis-Menten动力学。顶端/基础pH值(6.0 / 6.0、6.0 / 7.7和7.7 / 8.7)的变化对动力学影响不显着。相反,HG通量动力学仅在pH 7.7 / 8.7或具有中等亲和力的乳酸存在下获得。另外,从0到150μM的DL-MHA转运证明PC和MG中存在Na +依赖性高亲和力转运蛋白。最终,沿着鳟鱼肠道发现了两种不同的载体介导的DL-MHA转运机制:1)在PC和MG中:顶端转运受Na +调控,其中可能含有低亲和力和高亲和力转运体以及基底外侧转运主要是通过H +-独立的转运蛋白;2)在HG中:摄取由具有中等亲和力的Na +依赖性转运蛋白间接介导,而基底外侧出口在很大程度上受H +依赖性转运蛋白控制。最后,比较了两种主要的蛋氨酸饲料补充剂DL-MHA和DL-蛋氨酸(DL-Met),以了解其生物效率的差异。与DL-Met相比,PC和MG中DL-MHA的通量率仅约为42.2–66.0%,这表明DL-MHA的肠道转运低于DL-Met。



























 京公网安备 11010802027423号
京公网安备 11010802027423号